��Ŀ����
������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ��
��1��1molN2��g��������O2��g����Ӧ������NO2��g��������68kJ����______
��2��1molCu��s��������O2��g����Ӧ������CuO��s�����ų�157kJ����______
��3�����Ƿ���ʱ�����£�N2H4����ȼ�ϣ�1molN2H4��l����O2��g����ȼ�գ�����N2��g����H2O��l�����ų�622kJ������
______
��4����0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ______������֪��H2O��g��=H2O��l������H2=-44.0kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ�ų���������______kJ��
��1��1molN2��g��������O2��g����Ӧ������NO2��g��������68kJ����______
��2��1molCu��s��������O2��g����Ӧ������CuO��s�����ų�157kJ����______
��3�����Ƿ���ʱ�����£�N2H4����ȼ�ϣ�1molN2H4��l����O2��g����ȼ�գ�����N2��g����H2O��l�����ų�622kJ������
______
��4����0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ______������֪��H2O��g��=H2O��l������H2=-44.0kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ�ų���������______kJ��
��1��1molN2��g��������O2��g����Ӧ������NO2��g��������68kJ��������Ӧ����ʱ�ʱ�ֵΪ��ֵ�����Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2��g��+2O2��g��=2NO2��g����H=+68kJ?mol-1��
�ʴ�Ϊ��N2��g��+2O2��g��=2NO2��g����H=+68kJ?mol-1��
��2������ʱ�ʱ�ֵΪ��ֵ��1molCu��s��������O2��g����Ӧ������CuO��s�����ų�157kJ�������Ȼ�ѧ����ʽΪCu��s��+
O2��g��=CuO��s������H=-157kJ?mol-1��
�ʴ�Ϊ��Cu��s��+
O2��g��=CuO��s������H=-157kJ?mol-1��
��3��1molN2H4��l����O2��g����ȼ�գ�����N2��g����H2O��l�����ų�622kJ��������ʱ�ʱ�ֵΪ��ֵ�����Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2H4��g��+O2��g��=N2��g��+2H2O��l����H=-622kJ?mol-l��
�ʴ�Ϊ��N2H4��g��+O2��g��=N2��g��+2H2O��l����H=-622kJ?mol-l��
��4��0.3mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5KJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�2165KJ����������Ӧ���Ȼ�ѧ����ʽΪB2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��
B2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��
H2O��g��=H2O��l������H2=-44.0kJ/mol��
��-�ڡ�3�ã�B2H6��g��+3O2��g��=B2O3��s��+3H2O��g����H=-2033kJ/mol��11.2L����״������0.5mol��������ȫȼ��������̬ˮʱ�ų���������Ϊ2033kJ��0.5=1016.5kJ��
�ʴ�Ϊ��B2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��1016.5kJ��
�ʴ�Ϊ��N2��g��+2O2��g��=2NO2��g����H=+68kJ?mol-1��
��2������ʱ�ʱ�ֵΪ��ֵ��1molCu��s��������O2��g����Ӧ������CuO��s�����ų�157kJ�������Ȼ�ѧ����ʽΪCu��s��+
| 1 |
| 2 |
�ʴ�Ϊ��Cu��s��+
| 1 |
| 2 |
��3��1molN2H4��l����O2��g����ȼ�գ�����N2��g����H2O��l�����ų�622kJ��������ʱ�ʱ�ֵΪ��ֵ�����Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2H4��g��+O2��g��=N2��g��+2H2O��l����H=-622kJ?mol-l��
�ʴ�Ϊ��N2H4��g��+O2��g��=N2��g��+2H2O��l����H=-622kJ?mol-l��
��4��0.3mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5KJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�2165KJ����������Ӧ���Ȼ�ѧ����ʽΪB2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��
B2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��
H2O��g��=H2O��l������H2=-44.0kJ/mol��
��-�ڡ�3�ã�B2H6��g��+3O2��g��=B2O3��s��+3H2O��g����H=-2033kJ/mol��11.2L����״������0.5mol��������ȫȼ��������̬ˮʱ�ų���������Ϊ2033kJ��0.5=1016.5kJ��
�ʴ�Ϊ��B2H6��g��+3O2��g��=B2O3��s��+3H2O��l����H=-2165kJ/mol��1016.5kJ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
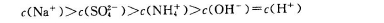
 �����κ��¶��¶����Է�����
�����κ��¶��¶����Է�����