��Ŀ����
��ҵ���ö����������ƴ��ᣬ��Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��2C4H10+5O2 4CH3COOH+2H2O������58�ֶ���Ϊԭ����ȡ���ᣬ��
4CH3COOH+2H2O������58�ֶ���Ϊԭ����ȡ���ᣬ����1����������Ҫ��״���µĿ��� m3���������O2��N2����������ֱ�Ϊ0.2��0.8����ͬʱ����ˮ �֣�
��2�������ɵĴ����ܽ������ɵ�ˮ�У����ô������������Ϊ %��
��3����ͬʱ������������Ϊ100%�ı�����m1�ֺ���������Ϊ50%�Ĵ���m2�֣���
 ���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ ����������ˮ����m1+m2= ��
���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ ����������ˮ����m1+m2= ��
���𰸡���������1����O2��Ҫ�����ʵ���Ϊxmol�����ݷ���ʽ�м������������ʵ�������������ڿ����еĺ���������Ҫ�����������
������ˮy�֣����ݷ���ʽ��������ˮ��������
��2�������ɴ���m�֣����ݷ���ʽ�������ɴ�����������������������Ķ�����㣮��3���������֪��m1+50%×m2=120�����貹��ˮy�֣���50%×m2=18+y������m2=36+2y������m1=120-18-y=102-y��
��������ˮ��50%×m2=18������m2=36����m1=120-50%×m2=102��
����⣺��1����O2��Ҫ�����ʵ���Ϊxmol����
2 C4H10+5O2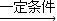 4CH3COOH+2H2O��
4CH3COOH+2H2O��
58×2g 5mol
58×106 xmol
����x=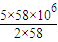 =2.5×106
=2.5×106
������Ҫ���������Ϊ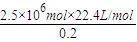 =2.8×108L=2.8×105m3��
=2.8×108L=2.8×105m3��
������ˮy�֣���
2 C4H10+5 O2 4CH3COOH+2H2O��
4CH3COOH+2H2O��
58×2g 18×2g
58�� y��
����y=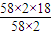 =18
=18
�ʴ�Ϊ��2.8×105��18��
��2�������ɴ���m�֣���
2 C4H10+5 O2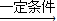 4CH3COOH+2H2O��
4CH3COOH+2H2O��
58×2g 4×60g
58�� m��
����m=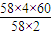 =120
=120
�ɣ�1���м����֪������ˮ18�֣�
���ɵĴ����ܽ������ɵ�ˮ�У����ô������������Ϊ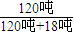 ×100%=86.96%��
×100%=86.96%��
�ʴ�Ϊ��86.96%��
��3���������֪��m1+50%×m2=120��
���貹��ˮy�֣���50%×m2=18+y������m2=36+2y������m1=120-18-y=102-y����102-y=x��36+2y������y=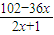 ��
��
��������ˮ��50%×m2=18������m2=36����m1=120-50%×m2=102������m1+m2=36+102=138��
�ʴ�Ϊ��y=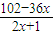 ��138��
��138��
������������ݷ���ʽ�ļ��㣬�Ѷ��еȣ��������ϴ���ϸ�ļ��㣬ּ�ڿ���ѧ�������ݴ��������÷���ʽ���м��㣮
������ˮy�֣����ݷ���ʽ��������ˮ��������
��2�������ɴ���m�֣����ݷ���ʽ�������ɴ�����������������������Ķ�����㣮��3���������֪��m1+50%×m2=120�����貹��ˮy�֣���50%×m2=18+y������m2=36+2y������m1=120-18-y=102-y��
��������ˮ��50%×m2=18������m2=36����m1=120-50%×m2=102��
����⣺��1����O2��Ҫ�����ʵ���Ϊxmol����
2 C4H10+5O2
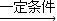 4CH3COOH+2H2O��
4CH3COOH+2H2O��58×2g 5mol
58×106 xmol
����x=
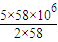 =2.5×106
=2.5×106������Ҫ���������Ϊ
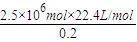 =2.8×108L=2.8×105m3��
=2.8×108L=2.8×105m3��������ˮy�֣���
2 C4H10+5 O2
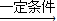 4CH3COOH+2H2O��
4CH3COOH+2H2O��58×2g 18×2g
58�� y��
����y=
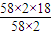 =18
=18�ʴ�Ϊ��2.8×105��18��
��2�������ɴ���m�֣���
2 C4H10+5 O2
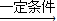 4CH3COOH+2H2O��
4CH3COOH+2H2O��58×2g 4×60g
58�� m��
����m=
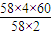 =120
=120�ɣ�1���м����֪������ˮ18�֣�
���ɵĴ����ܽ������ɵ�ˮ�У����ô������������Ϊ
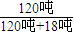 ×100%=86.96%��
×100%=86.96%���ʴ�Ϊ��86.96%��
��3���������֪��m1+50%×m2=120��
���貹��ˮy�֣���50%×m2=18+y������m2=36+2y������m1=120-18-y=102-y����102-y=x��36+2y������y=
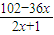 ��
����������ˮ��50%×m2=18������m2=36����m1=120-50%×m2=102������m1+m2=36+102=138��
�ʴ�Ϊ��y=
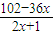 ��138��
��138��������������ݷ���ʽ�ļ��㣬�Ѷ��еȣ��������ϴ���ϸ�ļ��㣬ּ�ڿ���ѧ�������ݴ��������÷���ʽ���м��㣮

��ϰ��ϵ�д�
�����Ŀ
 4CH3COOH��2H2O
4CH3COOH��2H2O 4CH3COOH��2H2O
4CH3COOH��2H2O ���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ ���ú�x�Ĵ���ʽ��ʾy������������ˮ����m1��m2�� ��
���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ ���ú�x�Ĵ���ʽ��ʾy������������ˮ����m1��m2�� �� 4CH3COOH��2H2O
4CH3COOH��2H2O ���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ ���ú�x�Ĵ���ʽ��ʾy������������ˮ����m1��m2�� ��
���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ ���ú�x�Ĵ���ʽ��ʾy������������ˮ����m1��m2�� �� 4CH3COOH��2H2O
4CH3COOH��2H2O ���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ
���ú�x�Ĵ���ʽ��ʾy������������ˮ����m1��m2��
��
���貹��ˮy�֣���y��x�Ĺ�ϵʽΪ
���ú�x�Ĵ���ʽ��ʾy������������ˮ����m1��m2��
��