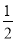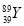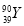ĢāÄæÄŚČŻ
ČēĶ¼¼×”¢ŅŅŹĒµē»ÆѧŹµŃé×°ÖĆ”£
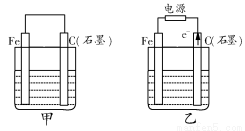
(1)Čō¼×”¢ŅŅĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠ±„ŗĶNaClČÜŅŗ”£
¢Ł¼×ÖŠŹÆÄ«°ōÉĻµÄµē¼«·“Ó¦Ź½ĪŖ__________________________________£»
¢ŚŅŅÖŠ×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________________________________£»
¢Ū½«ŹŖČóµÄµķ·Ū£KIŹŌÖ½·ÅŌŚŅŅÉÕ±ÉĻ·½£¬·¢ĻÖŹŌÖ½Ļȱ䥶ŗóĶŹÉ«£¬ÕāŹĒŅņĪŖ¹żĮæµÄCl2Ńõ»ÆĮĖÉś³ÉµÄI2”£
Čō·“Ó¦ÖŠCl2ŗĶI2µÄĪļÖŹµÄĮæÖ®±ČĪŖ5£ŗ1£¬ĒŅÉś³ÉĮ½ÖÖĖį£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________________”£
(2)Čō¼×”¢ŅŅĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠCuSO4ČÜŅŗ”£
¢Ł¼×ÖŠĢś°ōÉĻµÄµē¼«·“Ó¦Ź½ĪŖ____________________________________£»
¢ŚČē¹ūĘšŹ¼Ź±ŅŅÖŠŹ¢ÓŠ200 mL pH£½5µÄCuSO4ČÜŅŗ(25”ę)£¬Ņ»¶ĪŹ±¼äŗóČÜŅŗµÄpH±äĪŖ1£¬ČōŅŖŹ¹ČÜŅŗ»Öø“µ½µē½āĒ°µÄדĢ¬£¬æÉĻņČÜŅŗÖŠ¼ÓČė___________(ĢīŠ“ĪļÖŹµÄ»ÆѧŹ½)________g”£
(1)¢ŁO2£«2H2O£«4e£=4OH£
¢Ś2Cl££«2H2O 2OH££«H2”ü£«Cl2”ü
2OH££«H2”ü£«Cl2”ü
¢Ū5Cl2£«I2£«6H2O=10HCl£«2HIO3
(2)¢ŁFe£2e£=Fe2£«
¢ŚCuO(»ņCuCO3)””0.8(»ņ1.24)
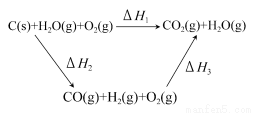
”¾½āĪö”æ(1)ČōĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠ±„ŗĶNaClČÜŅŗ£¬Ōņ£ŗ¢Ł¼××°ÖĆĪŖŌµē³Ų£¬ŹÆÄ«°ō×÷Õż¼«£¬O2·Åµē£¬µē¼«·“Ó¦ĪŖ£ŗO2£«2H2O£«4e£=4OH£”£¢ŚŅŅ×°ÖĆĪŖµē½ā³Ų£¬ŹÆÄ«°ōÉĻ·¢ÉśŃõ»Æ·“Ó¦£¬Feµē¼«²»²ĪÓė·“Ó¦£¬Ę䏵֏ÓėÓƶčŠŌµē¼«µē½āŹ³ŃĪĖ®ĻąĶ¬£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗ2Cl££«2H2O Cl2”ü£«H2”ü£«2OH£”£¢ŪCl2ŗĶI2µÄĪļÖŹµÄĮæÖ®±ČĪŖ5£ŗ1£¬Éś³ÉHClŗĶHIO3”£(2)ČōĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠCuSO4ČÜŅŗ£¬Ōņ£ŗ¢Ł¼××°ÖĆĪŖŌµē³Ų£¬Ģś×÷øŗ¼«£¬µē¼«·“Ó¦ĪŖ£ŗFe£2e£=Fe2£«”£¢ŚŅŅ×°ÖĆĪŖµē½ā³Ų£¬µē½ā·“Ó¦ĪŖ£ŗ2CuSO4£«2H2O
Cl2”ü£«H2”ü£«2OH£”£¢ŪCl2ŗĶI2µÄĪļÖŹµÄĮæÖ®±ČĪŖ5£ŗ1£¬Éś³ÉHClŗĶHIO3”£(2)ČōĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠCuSO4ČÜŅŗ£¬Ōņ£ŗ¢Ł¼××°ÖĆĪŖŌµē³Ų£¬Ģś×÷øŗ¼«£¬µē¼«·“Ó¦ĪŖ£ŗFe£2e£=Fe2£«”£¢ŚŅŅ×°ÖĆĪŖµē½ā³Ų£¬µē½ā·“Ó¦ĪŖ£ŗ2CuSO4£«2H2O 2Cu£«2H2SO4£«O2”ü£¬øł¾Żµē½ā·½³ĢŹ½£¬“Óµē½āÖŹČÜŅŗÖŠĪö³öCu£¬·Å³öO2£¬Ņņ“ĖÖ»Šč¼ÓČėCuO(»ņCuCO3)¼“æÉ»Öø“µ½µē½āĒ°µÄדĢ¬”£Óɵē½ā·“Ó¦Ź½æÉÖŖ£¬2H£«”«CuO”«CuCO3£¬µē½āŗón(H£«)£½0.1”Į0.2£½0.02(mol)£¬¹Źm(CuO)£½0.02”Į
2Cu£«2H2SO4£«O2”ü£¬øł¾Żµē½ā·½³ĢŹ½£¬“Óµē½āÖŹČÜŅŗÖŠĪö³öCu£¬·Å³öO2£¬Ņņ“ĖÖ»Šč¼ÓČėCuO(»ņCuCO3)¼“æÉ»Öø“µ½µē½āĒ°µÄדĢ¬”£Óɵē½ā·“Ó¦Ź½æÉÖŖ£¬2H£«”«CuO”«CuCO3£¬µē½āŗón(H£«)£½0.1”Į0.2£½0.02(mol)£¬¹Źm(CuO)£½0.02”Į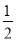 ”Į80£½0.8(g)£¬m(CuCO3)£½0.02”Į
”Į80£½0.8(g)£¬m(CuCO3)£½0.02”Į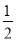 ”Į124£½1.24(g)”£
”Į124£½1.24(g)”£

 ·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø
·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø