��Ŀ����
��������Ҫ�ɷ�ΪFeS2�����ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧѧϰС���ij������ʯ��������ʵ��̽����
[ʵ��һ]�ⶨ��Ԫ�صĺ�����
��m1 g�û�������Ʒ��������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2
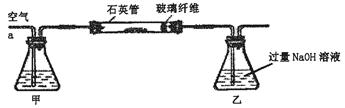
��Ӧ��������ƿ�е���Һ�������´�����
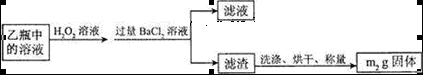
[ʵ���]�ⶨ��Ԫ�صĺ�����III���ⶨ��Ԫ�غ�����ʵ�鲽����ͼ��ʾ��

�������ۣ�
��1��I�У���ƿ����ʢ�Լ���____��Һ����ƿ�ڷ�����Ӧ�����ӷ���ʽΪ____��
��2��II�У���ƿ����H2O2��Һʱ��Ӧ�����ӷ���ʽΪ_____________________��
��3���û���������Ԫ�ص���������Ϊ_______________________��
��4��III�IJ�����У���Ҫ�õ����������ձ�������������ͷ�ι��⣬����____________________________________________��
��5��III�IJ�����У���ʾ�ζ��Ѵ��յ��������
��6����IJ���ܽ���������ƽ��ʵ�飬�������KMnO4��Һ����ֱ�Ϊ24.98mL��24.80mL��25.02mL��KMnO4����ԭΪMn2+���������������ݣ��ɼ�����û�������Ʒ��Ԫ�ص���������Ϊ ��
[ʵ��һ]�ⶨ��Ԫ�صĺ�����
��m1 g�û�������Ʒ��������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2+11O2
 2Fe2O3+8SO2
2Fe2O3+8SO2
��Ӧ��������ƿ�е���Һ�������´�����

|

�������ۣ�
��1��I�У���ƿ����ʢ�Լ���____��Һ����ƿ�ڷ�����Ӧ�����ӷ���ʽΪ____��
��2��II�У���ƿ����H2O2��Һʱ��Ӧ�����ӷ���ʽΪ_____________________��
��3���û���������Ԫ�ص���������Ϊ_______________________��
��4��III�IJ�����У���Ҫ�õ����������ձ�������������ͷ�ι��⣬����____________________________________________��
��5��III�IJ�����У���ʾ�ζ��Ѵ��յ��������
��6����IJ���ܽ���������ƽ��ʵ�飬�������KMnO4��Һ����ֱ�Ϊ24.98mL��24.80mL��25.02mL��KMnO4����ԭΪMn2+���������������ݣ��ɼ�����û�������Ʒ��Ԫ�ص���������Ϊ ��
��1���������ƣ����������أ���2�֣� SO2+2OH��=SO32��+H2O����2SO32��+O2=2SO42��δд���۷֣���2�֣�
��2��SO32��+H2O2=SO42��+H2O ��2�֣�
��3�� ��2�֣�
��2�֣�
��4��250ml����ƿ��2�֣�
��5�����һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ����2�֣�
��6�� ��
�� �����������𰸣�2�֣�
�����������𰸣�2�֣�
��2��SO32��+H2O2=SO42��+H2O ��2�֣�
��3��
 ��2�֣�
��2�֣���4��250ml����ƿ��2�֣�
��5�����һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ����2�֣�
��6��
 ��
�� �����������𰸣�2�֣�
�����������𰸣�2�֣������������1��Ϊ��ֹSO2���뵽��װ���У������������ƣ����������أ����գ���ƿ�е�������������Ӳ�ʲ������в����Ķ�����������Ӧ�����ӷ���ʽΪ��SO2+2OH��=SO32��+H2O��������ƿ��Ҳ������δ��ȫ��Ӧ����������Ҳ����2SO32��+O2=2SO42����2��II�У���ƿ����H2O2��Һʱ��Ӧ�����ӷ���ʽΪSO32��+H2O2=SO42��+H2O����3����ƿ�е���Һ����SO42����SO42���������Ȼ����е�Ba2+�������Ϸ���������Ӧ��SO42��+Ba2+=BaSO4����������m2gΪBaSO4����������й�ϵʽ���£�
FeS2��2SO42����2BaSO4
1mol 2mol
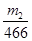

�ɴ˿�֪����Ԫ����FeS2�е����ʵ���Ϊ
 ������������
����������Ϊ��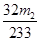 �����Ըû���������Ԫ�ص���������Ϊ
�����Ըû���������Ԫ�ص���������Ϊ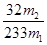 ��������4��ӦΪ250ml����ƿ����5����Ϊδ����KMnO4��Һ֮ǰ����Һ�к���Fe2+����ҺΪdz��ɫ��
��������4��ӦΪ250ml����ƿ����5����Ϊδ����KMnO4��Һ֮ǰ����Һ�к���Fe2+����ҺΪdz��ɫ�����������һ�θ��������Һ����ʱ����Һ��ɫͻ��Ϊ��ɫ������30s�ڲ���ɫ����֤������ζ��յ㡣
��6��������Ӧ�Ļ�ѧ����ʽΪ5Fe2++8H++MnO4-=Mn2++5Fe3++4H2O,Ȼ�����5Fe2+��MnO4-�й�ϵʽ�����
�û�������Ʒ��Ԫ�ص���������Ϊ
 ��
��
����㡣

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
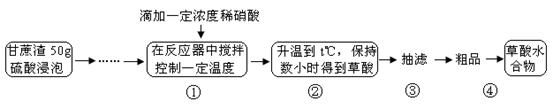
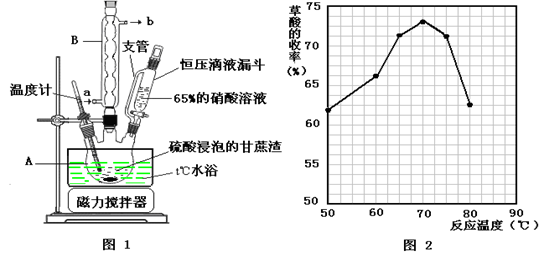 �����������Ϣ�ش��������⣺
�����������Ϣ�ش��������⣺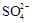 ʱ�����������ữ���ټ���
ʱ�����������ữ���ټ���