��Ŀ����
5��������������һ������ζ�İ�ɫ��ĩ����һ�����͵��������������������ĺϳɷ�ʽ���£�ԭ����CO��NH2��2+H2O2$\stackrel{30��}{��}$ CO��NH2��2•H2O2����������ͼ1��
��ش�
��1�����ݷ�Ӧԭ����CO��NH2��2•H2O2�����ڴ��ڵ���������AD��
A�����ۼ� B�����Ӽ�C�������� D�����
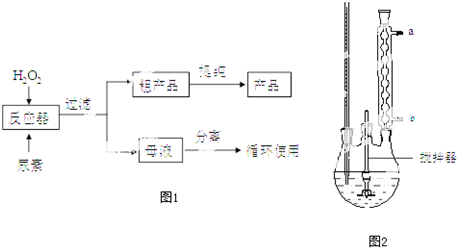
��2������ͼ�з�Ӧ����װ����ͼ2��ʾ�Ʋ�÷�Ӧ�ļ��ȷ�ʽ��30��ˮԡ������������ˮ����b��
�����룻����ѡ�õIJ����Dz������������ʻ����ʲ��ϵ�ԭ���ǹ����������������������ɵĽ��������ܴ���������ķֽ⣮
��3����ĸҺ�з����H2O2�����أ����õIJ�����B��
A������������ B����ѹ���ᾧC����Һ������ D����ѹ������ȡ
��4��Ϊ�ⶨ��Ʒ�л������ĺ�����������16%���൱��H2O234%������ȡ������Ʒ2.000g���ܽ⣬��250mL����ƿ�ж��ݣ�ȷ��ȡ25.00mL����ƿ�У�����1mL 6mol•L-1�����ᣬȻ����0.1 000mol•L-1KMnO4����Һ�ζ������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ8.000mL��KMnO4��Һ�����ز���Ӧ����
����ɲ���ƽ����ʽ����MnO4-+��H2O2+��H+=��Mn2++��H2O+��O2��
�ڱ�ʵ��KMnO4��Һ�ζ������в����ζ��ܵ�ͼʾ��ȷ����A�����ţ���

���ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õĻ���������ƫ�ߣ�����ƫ�ߡ�����ƫ�͡����䡱����
�۸��ݵζ��������ȷ����Ʒ�л���������������Ϊ16%��
���� ��1���ǽ���Ԫ��ԭ��֮�����γɹ��ۼ�������Ԫ����ǽ���Ԫ��ԭ��֮�����γɹ��ۼ�������֮����ڷ��»�����������������е�Oԭ�Ӻ����������е�Hԭ�����γ�������������ʣ�������������
��2��CO��NH2��2•H2O2���Ʊ��¶Ƚϵ�30�棬Ӧ����ˮԡ���ȣ�����װ��ͼ����������ˮӦ�ô��¿����룻����������Fe��Al��Ӧ�������������ӣ�
��3���������ʷе㲻ͬ��Һ��ķе��������ѹ���ı仯���仯�ķ����жϣ�
��4��������������ԭ��Ӧ�����غ�͵���غ㣬�����غ㣬ԭ���غ���ƽд�����ӷ���ʽ��
��KMnO4��Һ����ʽ�ζ���ʢ�ţ��ζ�ʱ��ת���������ݵζ��������������ݣ�c����V��=c�����V���⣬��������ԭ���DZ�Һ���ĵĶ��ٷ����жϣ�
������2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2����������������ʵ�������������Ϣ�����Ʒ�л�����������������
��� �⣺��1�����̷�����֪���ϳɹ��������������ù�����������ػ��Ϸ�Ӧ���ɹ��������أ���Ӧ�Ļ�ѧ����ʽΪ��CO��NH2��2+H2O2=CO��NH2��2•H2O2��CO��NH2��2��H2O2�����ڴ��ڹ��ۼ���CO��NH2��2������H2O2���Ӽ��������ͷ��»���������CO��NH2��2•H2O2�����ڴ��ڵ��������ǹ��ۼ��������
�ʴ�Ϊ��AD��
��2��CO��NH2��2•H2O2���Ʊ�CO��NH2��2+H2O2$\stackrel{30��}{��}$ CO��NH2��2•H2O2�¶Ƚϵ�30�棬CO��NH2��2•H2O2��45��ʱ�����ֽ⣬���Բ����þƾ���ֱ�Ӽ��ȣ�Ӧ����30��ˮԡ���ȣ�����װ��ͼ��֪ͼ2����Ϊ������ƿ������ˮӦ�ô��¿����룬��������Ч���ã�������ˮ���¿�b���룻����������Fe��Al��Ӧ�������������ӣ����������ӻ���ٹ�������ķֽ⣬���Խ��������������ʡ����ʲ��ϵģ�
�ʴ�Ϊ��30��ˮԡ��b�������������������������ɵĽ��������ܴ���������ķֽ⣻
��3��Һ��ķе���ָ��������ѹ�������ѹ��ʱ���¶ȣ����Һ��ķе��������ѹ���ı仯���仯�ģ������������ձý���ϵͳ��ѹ�����Ϳ��Խ���Һ��ķе㣮H2O2��ѹ�·е�108�棬���س�ѹ��169.6�棬��е�͵��ȷ��ڣ�����Һ̬��Ϊ��̬����ΪH2O2�����ֽ⣬���Դ�ĸҺ�з����H2O2������ʱ��Ӧʹ��Һ�ڽϵ��¶������������õIJ����Ǽ�ѹ����Ȼ��ᾧ��
�ʴ�Ϊ��B��
��4���ٷ�Ӧ�У�MnԪ�صĻ��ϼ۽�����5�ۣ���Ԫ�صĻ��ϼ����ߣ���-1�����ߵ�0�ۣ�1mol˫��ˮʧ������2mol��1mol����������ӵõ�����5mol�����ݵ����غ㣬��Ӧת�Ƶ�����10mol�����Ը���������ӵ�ǰ��ϵ����2��˫��ˮ��ǰ����ϵ����5�����ݵ���غ��Ԫ���غ㣬�õ��ķ���ʽΪ��2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
�ʴ�Ϊ��2��5��6��2��8��5O2��
��KMnO4��Һ����ʽ�ζ���ʢ�ţ��ζ�ʱ������ת������ֻ��ͼA���ϣ����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ�����ݷ�Ӧ�ĵ����ϵ��֪��5��c����V��=c�����V�����2����ñ�Һ���ƫ���ƫ�ߣ�
�ʴ�Ϊ��A��ƫ�ߣ�
�۳�ȡ������Ʒ2.000g���ܽ⣬��250mL����ƿ�ж��ݣ�ȷ��ȡ25.00mL����ƿ�У�����1mL 6mol/L�����ᣬȻ����0.1000mol/L KMnO4 ����Һ�ζ������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ8.00mL�����ݷ�Ӧ2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2��
2MnO4-����������5H2O2��
2 5
0.00800L��0.1000mol/L 0.02mol
250mL����ƿ�к���������0.2mol��
����������������=$\frac{0.2mol��34g/mol}{2g}$��100%=34%
������16%���൱��H2O234%�����������������=$\frac{16%��34%}{34%}$=16%��
�ʴ�Ϊ��16%��
���� ���⿼���������Ʊ�ʵ����̷����жϣ�װ��ͼ������Ӧ�ã��ζ�ʵ����̺ͼ���ķ���Ӧ�ã�Ϊ��Ƶ���㣬���շ����Ļ�ѧ��Ӧ��ʵ�����Ϊ���Ĺؼ������ط������������������Ŀ��飬ע����Ϣ����ѧ֪ʶ�Ľ�ϣ���Ŀ�ѶȽϴ�

| A�� | 3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+2CO2 | B�� | 2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+Al2O3 | ||
| C�� | NaHCO3+NaHSO4�TNa2SO4+CO2��+H2O | D�� | 2FeSO4$\frac{\underline{\;����\;}}{\;}$Fe2O3+SO2��+SO3�� |
| A�� | ��50mL��Ͳ������0.1000mo1•L-1̼������Һ | |
| B�� | 0.5mol O3��11.2L O3�����ķ�����һ����� | |
| C�� | ��������ΪNA��NO2��CO2��������к��е���ԭ����Ϊ2NA | |
| D�� | ���³�ѹ�£�22.4L��NO2��CO2������庬��2NA����ԭ�� |
| A�� | �����Ǻ���ȩ | B�� | ��ȩ�ͱ�ͪ | C�� | ��ȩ������ | D�� | ���Ǻ������� |
| A�� | �������ӵ�ֱ����1��100 nm֮�� | |
| B�� | ��������������� | |
| C�� | ���������ķ������뵰���ʺ��Ȼ��ƵĻ����Һ | |
| D�� | �������ȶ����ڵ���Ҫԭ���ǽ������Ӵ���� |
| A�� | HCO3-һ�����ܴ��������ڸ���Һ�� | |
| B�� | NH4+һ�����ܴ��������ڸ���Һ�� | |
| C�� | ����Һ��pHһ��Ϊ12 | |
| D�� | ����Һ��[H+]=[OH-] |
| A�� | �����£�����Ƭ����Ũ�����У�Fe+6HNO3��Ũ���TFe��NO3��3+3NO2��+3H2O | |
| B�� | ������ͭ�����ᷴӦ��H++OH-=H2O | |
| C�� | ���ø�ʴ������ӡˢ��·�壺Fe3++Cu=Fe2++Cu2+ | |
| D�� | ��Ũ�����м���ͭƬ��Cu+4H++2NO3-�TCu2++2NO2��+2H2O |