��Ŀ����
3��Cl2��HCl������Ҫ�Ļ���ԭ�ϣ����߿����ת������Ӧ���£���ӦA��H2��g��+Cl2��g��$\frac{\underline{\;��ȼ\;}}{\;}$2HCl��g������ӦB��4HCl+O2$?_{400��}^{CuO/CuCl_{2}}$2Cl2+2H2O��֪����ӦB�У�4molHCl���������ų�115.6kJ��������
��
��1��HCl�ĵ���ʽ��
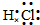 ��
����2��H2O�еĻ�ѧ�������ǹ��ۼ���
��3����ӦA�ġ�H=-183KJ/mol��
��4����ӦB���Ȼ�ѧ����ʽ��4HCl��g��+O2��g��?2Cl2��g��+2H2O��g����H=-115.6 KJ/mol��
��5���Ͽ�1molH-O����������ԼΪ462.9kJ��
���� ��1��HCl���ڹ��ۻ�������������Ӽ�����������ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�
��2��ˮ�ǹ��ۻ������ԭ�Ӻ�������ԭ���γ��������ۼ���
��3����ӦA�У��ʱ�=��Ӧ��ϼ���������-�������γɻ�ѧ���ų��������㣻
��4����ӦB�У�2mol HCl���������ų�115.6kJ�������������Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�Ͷ�Ӧ�ʱ�д����
��5�����ݷ�ӦB�У�4mol HCl���������ų�115.6kJ���������ʱ�=��Ӧ��ϼ���������-�������γɻ�ѧ���ų��������㣮
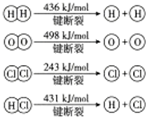
��� �⣺��1��HCl���ڹ��ۻ�������������Ӽ�����������ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�HCl����ʽΪ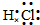 ���ʴ�Ϊ��
���ʴ�Ϊ��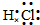 ��
��
��2��ˮ�ǹ��ۻ������ԭ�Ӻ�������ԭ���γ��������ۼ����ʴ�Ϊ�����ۼ���
��3����ӦA�У��ʱ�=��Ӧ��ϼ���������-�������γɻ�ѧ���ų�����=436+243-2��431=-183KJ/mol���ʴ�Ϊ��-183KJ/mol��
��4�����ݷ�ӦB�У�4mol HCl���������ų�115.6kJ����������Ӧ���Ȼ�ѧ����ʽΪ��4HCl��g��+O2��g��?2Cl2��g��+2H2O��g����H=-115.6 KJ/mol��
�ʴ�Ϊ��4HCl��g��+O2��g��?2Cl2��g��+2H2O��g����H=-115.6 KJ/mol
��5���ʱ�=��Ӧ��ϼ���������-�������γɻ�ѧ���ų�������4HCl��g��+O2��g��?2Cl2��g��+2H2O��g����H=-115.6 KJ/mol��
4��E��H-Cl��+498-[243��2+4��E��H-O��]=-115.6���õ�4��E��H-O��-4��431=498-486+115.6=127.6
E��H-O��=462.9��
�ʴ�Ϊ��462.9��
���� ���⿼���˵���ʽ����д���Ȼ�ѧ����ʽ��д����ѧ�����ܺ��ʱ��ϵ���㣬��Ŀ�Ѷ��еȣ�

| A�� | ���³�ѹ�£�1.06g Na2CO3���е�Na+������Ϊ0.02NA | |
| B�� | ���³�ѹ�£�32g O2��O3�Ļ����������ԭ����Ϊ2NA | |
| C�� | ��״���£�22.4L�������еķ�����ԼΪNA | |
| D�� | 500mL0.2mol•L-1BaCl2��Һ��Cl-Ũ��Ϊ0.2 mol•L-1 |
| A�� | H2��D2��T2��Ϊͬλ�� | |
| B�� | ����3�к��أ�������3������� | |
| C�� | ��������ͬ��14N16O��12C16O��������ͬ | |
| D�� | Na2O��2�����Ӿ�����ͬ�ĵ��Ӳ�ṹ |
| A�� | 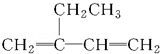 2-�һ�-1��3-����ϩ 2-�һ�-1��3-����ϩ | B�� | 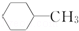 �ױ� �ױ� | ||
| C�� | CH3CH2CH2CH2OH ���� | D�� | HOCH2CH2CH2OH 1��3-������ |
| A�� | CH3CH3+Cl2$\stackrel{��}{��}$CH3CH2Cl+HCl | B�� | 2CH3CHO+O2$��_{��}^{����}$2CH3COOH | ||
| C�� |  +Br2$\stackrel{Fe}{��}$ +Br2$\stackrel{Fe}{��}$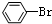 +HBr +HBr | D�� |  +HNO3$��_{50-60��}^{Ũ����}$ +HNO3$��_{50-60��}^{Ũ����}$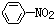 +H2O +H2O |
| A�� | PbO2�ǵ�ص����� | |
| B�� | ������ӦʽΪ��Pb+SO42--2e-�TPbSO4 | |
| C�� | PbO2�õ��ӣ������� | |
| D�� | ��طŵ�ʱ����ҺpH���� |
| A�� | �Ȼ�ͭ��Һ�����۷�Ӧ��Cu2++Fe�TFe3++Cu | |
| B�� | Ba��OH��2��Һ�еμ�NaHSO4��Һ�����ԣ�Ba2++2OH-+2H++SO42-�TBaSO4��+2H2O | |
| C�� | ��NaͶ�뵽CuSO4��Һ��2Na+Cu2+�T2Na++Cu | |
| D�� | �ô����ܽ�CaCO3��CaCO3+2H+�TCa2++H2O+CO2�� |