��Ŀ����
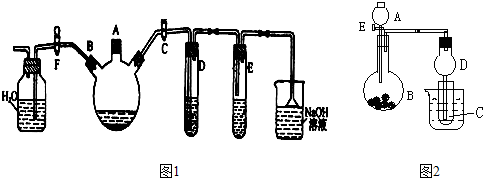
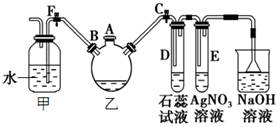
ʵ�����Ʊ��屽������ͼ��ʾװ�á�
��ش��������⣺
��1���ر�F�У���C�У���װ����������������ƿ����A�ڼ���ҺBr2���ټ���������м����סA�ڣ���������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��
�� ��
��2���Թ�D��E�ڳ��ֵ���������Ϊ �� ��
��3����������ƿ�еķ�Ӧ���е���������ð��ʱ��F�У��ر�C�У����Կ����������� �����ɶ�ѡ��
A�����е�ˮ����������
B�������д�������ð��
C�����е���Һ�������
D��������Һ���ֲַ�����
��4����Ҫ��ȥ�屽�л��е�Br2����ѡ���Լ�_________��
A���ƾ� B��NaOH��Һ C��CCl4 D����
��10�֣���1��2Fe+3Br2��2FeBr3
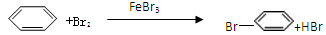
��2��D��ʯ����Һ��죬E�г��ֵ���ɫ������ ��3��AD ��4��B
��������
�����������1�����ǻ��õĽ������͵����巴Ӧ�����廯�������廯���������£�����Һ�巽ʽȡ����Ӧ�����屽����Ӧ�ķ���ʽ�ֱ���2Fe+3Br2��2FeBr3��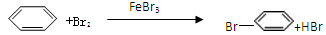 ��
��
��2�����ɵ��廯������ˮ�����ԣ�����������Ӧ���ɵ���ɫ�����廯������ʵ��������D��ʯ����Һ��죬E�г��ֵ���ɫ������
��3�������廯�⼫������ˮ�����������ʵ���������Ϊ���ɵ��屽������ˮ���ܶȴ���ˮ�ģ���ʵ���й��̵��������Ǽ��е�ˮ���������С�������Һ���ֲַ�������ѡAD��
��4��ѡ��ACD���屽�ǻ��ܻ����ܵģ�����ѡ�������ܰ�����������Һ���գ����屽���������Ʋ���Ӧ��������ˮ��������ȷ�Ĵ�ѡB��
���㣺�����屽�Ʊ����й�ʵ���ж�
��������ѧʵ�鳣��������ʹ�÷����ͻ�ѧʵ����������ǽ��л�ѧʵ��Ļ������Ի�ѧʵ��Ŀ����벻����ѧʵ��Ļ������������Ը��������Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������

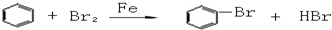
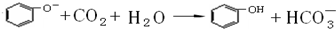
 CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3