��Ŀ����
������ʵ������У���������
A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170��
B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ
C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ��
D�������еμ�����ϡ��ˮ���������������鱽��
E����ҵ�ƾ���ȡ��ˮ�ƾ�ʱ���ȼ���ʯ��Ȼ������������뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
��ʵ������ͼ1��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ��
��1���ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��
 ��
��
��2��D�Թ���װ����
��3��E�Թ���װ����
��4����������ƿ�еķ�Ӧ��������ʱ����ʱ�������Լ��٣�����F�������ر�C���������Կ�����������
��5����һ���õ����屽��Ҫ�����²������ƣ�
a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ
��ȷ�IJ���˳����
������ͼ2��ʾװ�ý���ʵ�飬��A��μ���B�У�
��1����BΪNa2CO3��ĩ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�����ӷ���ʽ��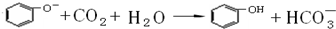
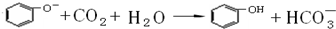 ��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�����
��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�����
��2����B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��
AC
AC
A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ���ʹҺ���¶�Ѹ������170��
B����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ�����ã���Һ��ֲ�μ���������Һ
C����ͭ˿�������״���ھƾ����ϼ��ȱ�ں�����������ˮ�Ҵ��У�����Ҵ�����Ϊ��ȩ��ʵ��
D�������еμ�����ϡ��ˮ���������������鱽��
E����ҵ�ƾ���ȡ��ˮ�ƾ�ʱ���ȼ���ʯ��Ȼ������������뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ�¶�
��ʵ������ͼ1��ʾװ���Ʊ��屽������֤�÷�Ӧ��ȡ����Ӧ��
��1���ر�F��������C��������װ��������������ƿ����A�ڼ��������壬�ټ���������м����סA�ڣ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��


��2��D�Թ���װ����
CCl4
CCl4
�������������ջӷ����ı���������
���ջӷ����ı���������
����3��E�Թ���װ����
AgNO3��Һ
AgNO3��Һ
��E�Թ��ڳ��ֵ�����Ϊ���ܿڲ����������������ɵ���ɫ����
���ܿڲ����������������ɵ���ɫ����
����4����������ƿ�еķ�Ӧ��������ʱ����ʱ�������Լ��٣�����F�������ر�C���������Կ�����������
ˮ������������ƿ��
ˮ������������ƿ��
����5����һ���õ����屽��Ҫ�����²������ƣ�
a���� bˮϴ�� c�ø������� d 10%NaOH��Һϴ�ӣ� eˮϴ
��ȷ�IJ���˳����
bdeca����edbca��
bdeca����edbca��
������ͼ2��ʾװ�ý���ʵ�飬��A��μ���B�У�
��1����BΪNa2CO3��ĩ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�����ӷ���ʽ��
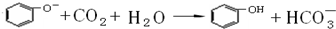
��Һ�ɻ��DZ����
��Һ�ɻ��DZ����
����2����B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��
Ũ��ˮ
Ũ��ˮ
�������ƣ���C��AgNO3
AgNO3
���ѧʽ��������ȩ�Ļ�Ϻ���Һ�з�Ӧ�Ļ�ѧ����ʽ��CH3CHO+2Ag��NH3��2OH CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3
 CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3CH3CHO+2Ag��NH3��2OH CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3
������D�ڴ�ʵ���е������� CH3COONH4+H2O+2Ag��+3NH3
CH3COONH4+H2O+2Ag��+3NH3��ֹ����
��ֹ����
��
��������A��Ũ��������������ˮ������Ƭ��ֹ���У������¶�170�棻
B��ˮ���Ӧ���������ԣ�
C���Ҵ���CuO����Ϊ��ȩ��
D��������Ũ��ˮ��Ӧ��
E���������ʵĻ�������Ҫ�¶ȼƲⶨ�¶ȣ�
��1�������巴Ӧ�����屽��
��2��D���ջӷ����ı����壻
��3��E����HBr�����ɣ�
��4����F�������ر�C������ˮ�ᷢ��������
��5�����屽�ᴿ����ˮϴ����NaOH���������
��1��C�����ɱ��ӳ������¶����ߣ��ܽ������
��2��B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��Ϊ������C��Ϊ��������Һ����ȩ��������Һ��Ӧ����Ag��D������װ�ÿɷ�ֹ������
B��ˮ���Ӧ���������ԣ�
C���Ҵ���CuO����Ϊ��ȩ��
D��������Ũ��ˮ��Ӧ��
E���������ʵĻ�������Ҫ�¶ȼƲⶨ�¶ȣ�
��1�������巴Ӧ�����屽��
��2��D���ջӷ����ı����壻
��3��E����HBr�����ɣ�
��4����F�������ر�C������ˮ�ᷢ��������
��5�����屽�ᴿ����ˮϴ����NaOH���������
��1��C�����ɱ��ӳ������¶����ߣ��ܽ������
��2��B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��Ϊ������C��Ϊ��������Һ����ȩ��������Һ��Ӧ����Ag��D������װ�ÿɷ�ֹ������
����⣺��A��Ũ��������������ˮ������Ƭ��ֹ���У������¶�170�棬ʵ���������A��ȷ��
B��ˮ���Ӧ���������ԣ���ˮ���Ϊ���ԣ���B����
C���Ҵ���CuO����Ϊ��ȩ��ʵ��Ϊ������ʵ�飬������������C��ȷ��
D��������ϡ��ˮ��Ӧ���ɵ����屽�����ڱ��ӣ����ܹ۲쵽��ɫ������Ӧ��Ũ��ˮ��Ӧ����D����
E���������ʵĻ�������Ҫ�¶ȼƲⶨ�¶ȣ���E����
�ʴ�Ϊ��AC��
��1�������巴Ӧ�����屽���÷�ӦΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��D���ջӷ����ı����壬��D��ΪCCl4������Ϊ���ջӷ����ı��������壬�ʴ�Ϊ��CCl4�����ջӷ����ı��������壻
��3��E����HBr�����ɣ���E��װAgNO3��Һ������Ϊ���ܿڲ����������������ɵ���ɫ�������ʴ�Ϊ��AgNO3��Һ�����ܿڲ����������������ɵ���ɫ������
��4����F�������ر�C�������۲쵽ˮ������������ƿ�У��ʴ�Ϊ��ˮ������������ƿ�У�
��5�����屽�ᴿ����ˮϴ����NaOH�����������˳��Ϊbdeca ����edbca�����ʴ�Ϊ��bdeca ����edbca����
��1��C�����ɱ��ӳ������÷�ӦΪ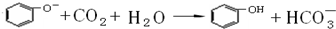 �������¶�ʱ�����ܽ��������۲쵽��Һ�ɻ��DZ���壬
�������¶�ʱ�����ܽ��������۲쵽��Һ�ɻ��DZ���壬
�ʴ�Ϊ��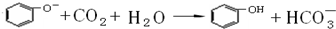 ����Һ�ɻ��DZ���壻
����Һ�ɻ��DZ���壻
��2��B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��Ϊ������C��Ϊ��������Һ����ȩ��������Һ��Ӧ����Ag��������ӦΪCH3CHO+2Ag��NH3��2OH CH3COONH4+H2O+2Ag��+3NH3��D������װ�ÿɷ�ֹ������
CH3COONH4+H2O+2Ag��+3NH3��D������װ�ÿɷ�ֹ������
�ʴ�Ϊ��Ũ��ˮ��AgNO3��CH3CHO+2Ag��NH3��2OH CH3COONH4+H2O+2Ag��+3NH3����ֹ������
CH3COONH4+H2O+2Ag��+3NH3����ֹ������
B��ˮ���Ӧ���������ԣ���ˮ���Ϊ���ԣ���B����
C���Ҵ���CuO����Ϊ��ȩ��ʵ��Ϊ������ʵ�飬������������C��ȷ��
D��������ϡ��ˮ��Ӧ���ɵ����屽�����ڱ��ӣ����ܹ۲쵽��ɫ������Ӧ��Ũ��ˮ��Ӧ����D����
E���������ʵĻ�������Ҫ�¶ȼƲⶨ�¶ȣ���E����
�ʴ�Ϊ��AC��
��1�������巴Ӧ�����屽���÷�ӦΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��D���ջӷ����ı����壬��D��ΪCCl4������Ϊ���ջӷ����ı��������壬�ʴ�Ϊ��CCl4�����ջӷ����ı��������壻
��3��E����HBr�����ɣ���E��װAgNO3��Һ������Ϊ���ܿڲ����������������ɵ���ɫ�������ʴ�Ϊ��AgNO3��Һ�����ܿڲ����������������ɵ���ɫ������
��4����F�������ر�C�������۲쵽ˮ������������ƿ�У��ʴ�Ϊ��ˮ������������ƿ�У�
��5�����屽�ᴿ����ˮϴ����NaOH�����������˳��Ϊbdeca ����edbca�����ʴ�Ϊ��bdeca ����edbca����
��1��C�����ɱ��ӳ������÷�ӦΪ
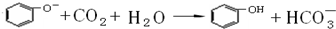 �������¶�ʱ�����ܽ��������۲쵽��Һ�ɻ��DZ���壬
�������¶�ʱ�����ܽ��������۲쵽��Һ�ɻ��DZ���壬�ʴ�Ϊ��
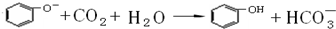 ����Һ�ɻ��DZ���壻
����Һ�ɻ��DZ���壻��2��B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�E��Ȼ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��Ϊ������C��Ϊ��������Һ����ȩ��������Һ��Ӧ����Ag��������ӦΪCH3CHO+2Ag��NH3��2OH
 CH3COONH4+H2O+2Ag��+3NH3��D������װ�ÿɷ�ֹ������
CH3COONH4+H2O+2Ag��+3NH3��D������װ�ÿɷ�ֹ�������ʴ�Ϊ��Ũ��ˮ��AgNO3��CH3CHO+2Ag��NH3��2OH
 CH3COONH4+H2O+2Ag��+3NH3����ֹ������
CH3COONH4+H2O+2Ag��+3NH3����ֹ���������������⿼�黯ѧʵ�鷽�������ۣ������л�������ʼ�ʵ��Ŀ��飬�漰֪ʶ��϶࣬�ۺ���ǿ���ϺõĿ���ѧ����������������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
�����Ŀ
����ʵ���������ȷ���ǣ�������
| A����Һʱ��������Ȼ�̼��Һ�ӷ�Һ©���Ͽ����� | B��Ϊ����Na2CO3��Һ���Ƿ��������NaOH����������Һ�еμ�CaCl2��Һ���������ٵμӼ��η�̪��Һ�۲���Һ�Ƿ��� | C������ƿ��©�IJ����ǣ�������ƿ��ע��������ˮ�����ϲ���ƿ����������ָ��סƿ�ף�����ʳָ��סƿ�����������ã��۲��Ƿ�©ˮ | D���������ʱ��Ӧʹ�¶ȼ�ˮ�������Һ����ⶨҺ����¶� |
����ʵ���������ȷ���ǣ�������
| A����ȡ�屽������м����ˮ������ϼ��� | B��ʵ������ȡ���������ȼ���Ũ���ᣬ�ټӱ���������Ũ���� | C������ױ��ͱ�����ױ��ͱ��зֱ��������KMnO4��Һ�����۲��Ƿ���ɫ | D������±�����е�±ԭ�ӣ�����NaOH��Һ���ȣ�Ȼ�����ϡ��������Һ�����ԣ��ټ�AgNO3��Һ���۲��������ɫ |