��Ŀ����
��8�֣���2008����ҹ��Ϸ������ı�ѩ�ֺ��У�ʹ����һ����ѩ��������Ҫ�ɷֵĻ�ѧʽΪXY2��X��YΪ���ڱ�ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1 molXY2����54 mol���ӡ�
��1������ѩ���Ļ�ѧʽ�� ��X����Ԫ���γɵĻ�������� ��
��2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D ��Y���ڣ���D������
��Y���ڣ���D������ �ṹʾ��ͼ�� ��D��E���γ�
�ṹʾ��ͼ�� ��D��E���γ� һ�ַǼ��Է��ӣ��÷��ӵĽṹʽΪ ��D������Ԫ�ص��⻯���У��е���͵��� ��
һ�ַǼ��Է��ӣ��÷��ӵĽṹʽΪ ��D������Ԫ�ص��⻯���У��е���͵��� ��
��3��Ԫ��W��Yͬ���ڣ��䵥����ԭ�Ӿ��壻Ԫ��Z�ĵ��ʷ���Z2����3�����۽���W��Z���γ�һ���������ǽ������ϣ��仯ѧʽ�� .
��4��Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R2��NaOH��Һ��Ӧ�IJ���֮һ��OR2���÷�Ӧ�����ӷ���ʽΪ ��
��1������ѩ���Ļ�ѧʽ�� ��X����Ԫ���γɵĻ�������� ��
��2��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D
 ��Y���ڣ���D������
��Y���ڣ���D������ �ṹʾ��ͼ�� ��D��E���γ�
�ṹʾ��ͼ�� ��D��E���γ� һ�ַǼ��Է��ӣ��÷��ӵĽṹʽΪ ��D������Ԫ�ص��⻯���У��е���͵��� ��
һ�ַǼ��Է��ӣ��÷��ӵĽṹʽΪ ��D������Ԫ�ص��⻯���У��е���͵��� ����3��Ԫ��W��Yͬ���ڣ��䵥����ԭ�Ӿ��壻Ԫ��Z�ĵ��ʷ���Z2����3�����۽���W��Z���γ�һ���������ǽ������ϣ��仯ѧʽ�� .
��4��Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R2��NaOH��Һ��Ӧ�IJ���֮һ��OR2���÷�Ӧ�����ӷ���ʽΪ ��
��1��CaCl2��
(2)
S=C=S��H2S��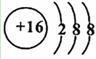
(3)Si3N4��
(4)2F2+2OH��=2F��+OF2+H2O��
(2)
S=C=S��H2S��
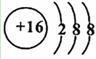
(3)Si3N4��
(4)2F2+2OH��=2F��+OF2+H2O��
��

��ϰ��ϵ�д�
�����Ŀ