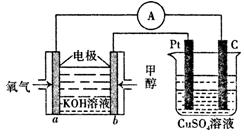
��Ŀ����
��֪����25ʱH2O?H++OH-��KW=10-14��CH3COOH?H++CH3COO-��Ka=1.8×10-5��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH���ı�ֵ______�������С�����䡱��
��2��������ˮ������ӷ���ʽΪ______���������¶�ʱ��C��OH-����______���������С�������䡱����
��3��0.5mol?L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д����Ƶı�ֵ��Ϊa��1mol?L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵΪ______��a��b�Ĺ�ϵΪ______������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳����______��
��5�������������������Һ��Ϻ�pH��7����c��Na+��______ c��CH3COO-��������ڡ�����С�ڡ����ڡ�����
��6������pH=3��HA��ҺV1mL��pH=11��NaOH{��ҺV2 mL����϶��ã�������˵������ȷ����______��
A������Ӧ����Һ�����ԣ���c��H+��+c��OH-��=2×10-7mol?L-1
B����V1=V2����Ӧ����ҺpHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2
��7����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ0.01mol?L-1�������м����������ƺ�����Һ��C��OH-��Ϊ2.2×10-5mol?L-1������������Һ����仯���������ֽ���������______�����ɳ�����ԭ����______
��KSP[Mg��OH��2]=1.8×10-11��KSP[Zn��OH��2]=1.2×10-17��KSP[Cd��OH��2]=2.5×10-14��
��8��ȡ10mL 0.5mol?L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��=______��
���𰸡���������1�����ݴ����ƹ����ƽ��CH3COOH?CH3COO-+H+��Ӱ�������
��2����������ӽ��ˮ��������������ɴ��ᣬ��Ӧ�ǿ���ģ���������ӵ�ˮ�������ȷ�Ӧ��
��3����������Һ�����ʵ���Ũ��Խ��ˮ��̶�ԽԽС��������Һ������������Ũ��Խ����Һ��PHԽ��
��4����������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ�ǡ������CH3COONa��Ϊǿ�������Σ���Һ�ʼ��ԣ�
��5��pH��7��Һ��ʾ���ԣ���Һ�������Ӵ�������������Ũ�ȣ����������Ũ�ȴ���������Ũ�ȣ�
��6��pH=3��HA��Һ��������Ũ�Ⱥ�pH=11��NaOH��Һ�е�������������ȣ�������������ʣ�����������ǿ����ʣ�������Щ��ѡ������жϣ�
��7���������ӵ�Ũ�Ⱥ�����������Ũ�ȣ�������������ӵ����ӻ���Ȼ�����KSP[Mg��OH��2]=1.8×10-11��KSP[Zn��OH��2]=1.2×10-17��KSP[Cd��OH��2]=2.5×10-14�����ж��Ƿ����ɳ�����
��8������10mL 0.5mol?L-1������Һ������������ӵ����ʵ���Ũ�ȣ���Һϡ�ͣ����������ʵ������䣬�����ϡ�ͺ���Һ��������Ũ�ȣ�������Һ��ˮ����������ӵ�����Һ�����������ӵ�Ũ�ȣ�
����⣺��1��������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH?CH3COO-+H+�����ƶ���C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С���ʴ�Ϊ����С��
��2��������ˮ������ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-���÷�Ӧ�����ȷ�Ӧ���¶����ߣ���Һ������������Ũ������
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-������
��3�����ڴ�������Һ�У������Ƶ�Ũ��Խ��ˮ��̶�ԽС��������Һ�е����������ӵ����ʵ���Ũ�ȷ���Խ����Һ��PHԽ������mС��n��a����b��
�ʴ�Ϊ��С�ڣ����ڣ�
��4������Ϊ������ʣ���������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ�ǡ������CH3COONa��Ϊǿ�������Σ���Һ�ʼ��ԣ�c��OH-����c��H+������Һ�д��ڣ�c��CH3COO-��+c��OH-��=c��Na+��+c��H+������c��Na+����c��CH3COO-����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
��5��������Һ��PHС��7����Һ�����������ʵ���Ũ�ȴ������������ӵ�Ũ�ȣ���������Ҫ�Ǵ������ģ����Դ��������Ũ�ȴ���������Ũ�ȣ�
�ʴ�Ϊ��С�ڣ�
��6��A������Ӧ����Һ�����ԣ���Һ��������Ũ��=����������Ũ��=1×10-7mol?L-1c��H+��+c��OH-��=2×10-7mol?L-1����A˵����ȷ��
B��������������ʲ��ֵ��룬�����Ũ�ȴ����������Ƶ�Ũ�ȣ���V1=V2����Ӧ����ҺpHһ��С��7����B˵������
C������Ӧ����Һ�����ԣ����ڴ���Ũ�ȴ�����������Ũ�ȣ���V1��V2����C˵����ȷ��
D������Ӧ����Һ�ʼ��ԣ�����V1=V2��Һ��ʾ���ԣ�����V1һ��С��V2����D˵����ȷ��
��ѡ��BC��
��7����Һ������������Ũ���ǣ�[OH-]=2.2×10-5mol?L-1������[M2+][OH-]2=5×10-12��mol?L-1��3��
����5×10-12С��KSP[Mg��OH��2]=1.8×10-11��û��������þ�������ɣ�
����5×10-12����KSP[Zn��OH��2]=1.2×10-17����������п�������ɣ�
����5×10-12����KSP[Cd��OH��2]=2.5×10-14����Cd��OH��2�������ɣ�
�ʴ�Ϊ��Cd2+��Zn2+��
��8��ϡ�ͺ���Һ�е�������Ũ���ǣ�c��H+��= =0.01mol/L������������Һ�У�ˮ�����������Ũ�ȵ�����Һ�е����������ӵ�Ũ�ȣ�����Һ����ˮ�������c��H+��=1×10-12��
=0.01mol/L������������Һ�У�ˮ�����������Ũ�ȵ�����Һ�е����������ӵ�Ũ�ȣ�����Һ����ˮ�������c��H+��=1×10-12��
�ʴ�Ϊ��1×10-12��
���������⿼��������ʵĵ���ƽ�⡢����ˮ�⡢����Ũ�ȱȽϡ���ҺPHֵ���жϵȣ��漰����Ŀ�����ϴ����Ѷ��еȣ�
��2����������ӽ��ˮ��������������ɴ��ᣬ��Ӧ�ǿ���ģ���������ӵ�ˮ�������ȷ�Ӧ��
��3����������Һ�����ʵ���Ũ��Խ��ˮ��̶�ԽԽС��������Һ������������Ũ��Խ����Һ��PHԽ��
��4����������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ�ǡ������CH3COONa��Ϊǿ�������Σ���Һ�ʼ��ԣ�
��5��pH��7��Һ��ʾ���ԣ���Һ�������Ӵ�������������Ũ�ȣ����������Ũ�ȴ���������Ũ�ȣ�
��6��pH=3��HA��Һ��������Ũ�Ⱥ�pH=11��NaOH��Һ�е�������������ȣ�������������ʣ�����������ǿ����ʣ�������Щ��ѡ������жϣ�
��7���������ӵ�Ũ�Ⱥ�����������Ũ�ȣ�������������ӵ����ӻ���Ȼ�����KSP[Mg��OH��2]=1.8×10-11��KSP[Zn��OH��2]=1.2×10-17��KSP[Cd��OH��2]=2.5×10-14�����ж��Ƿ����ɳ�����
��8������10mL 0.5mol?L-1������Һ������������ӵ����ʵ���Ũ�ȣ���Һϡ�ͣ����������ʵ������䣬�����ϡ�ͺ���Һ��������Ũ�ȣ�������Һ��ˮ����������ӵ�����Һ�����������ӵ�Ũ�ȣ�
����⣺��1��������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH?CH3COO-+H+�����ƶ���C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С���ʴ�Ϊ����С��
��2��������ˮ������ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-���÷�Ӧ�����ȷ�Ӧ���¶����ߣ���Һ������������Ũ������
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-������
��3�����ڴ�������Һ�У������Ƶ�Ũ��Խ��ˮ��̶�ԽС��������Һ�е����������ӵ����ʵ���Ũ�ȷ���Խ����Һ��PHԽ������mС��n��a����b��
�ʴ�Ϊ��С�ڣ����ڣ�
��4������Ϊ������ʣ���������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ�ǡ������CH3COONa��Ϊǿ�������Σ���Һ�ʼ��ԣ�c��OH-����c��H+������Һ�д��ڣ�c��CH3COO-��+c��OH-��=c��Na+��+c��H+������c��Na+����c��CH3COO-����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
��5��������Һ��PHС��7����Һ�����������ʵ���Ũ�ȴ������������ӵ�Ũ�ȣ���������Ҫ�Ǵ������ģ����Դ��������Ũ�ȴ���������Ũ�ȣ�
�ʴ�Ϊ��С�ڣ�
��6��A������Ӧ����Һ�����ԣ���Һ��������Ũ��=����������Ũ��=1×10-7mol?L-1c��H+��+c��OH-��=2×10-7mol?L-1����A˵����ȷ��
B��������������ʲ��ֵ��룬�����Ũ�ȴ����������Ƶ�Ũ�ȣ���V1=V2����Ӧ����ҺpHһ��С��7����B˵������
C������Ӧ����Һ�����ԣ����ڴ���Ũ�ȴ�����������Ũ�ȣ���V1��V2����C˵����ȷ��
D������Ӧ����Һ�ʼ��ԣ�����V1=V2��Һ��ʾ���ԣ�����V1һ��С��V2����D˵����ȷ��
��ѡ��BC��
��7����Һ������������Ũ���ǣ�[OH-]=2.2×10-5mol?L-1������[M2+][OH-]2=5×10-12��mol?L-1��3��
����5×10-12С��KSP[Mg��OH��2]=1.8×10-11��û��������þ�������ɣ�
����5×10-12����KSP[Zn��OH��2]=1.2×10-17����������п�������ɣ�
����5×10-12����KSP[Cd��OH��2]=2.5×10-14����Cd��OH��2�������ɣ�
�ʴ�Ϊ��Cd2+��Zn2+��
��8��ϡ�ͺ���Һ�е�������Ũ���ǣ�c��H+��=
 =0.01mol/L������������Һ�У�ˮ�����������Ũ�ȵ�����Һ�е����������ӵ�Ũ�ȣ�����Һ����ˮ�������c��H+��=1×10-12��
=0.01mol/L������������Һ�У�ˮ�����������Ũ�ȵ�����Һ�е����������ӵ�Ũ�ȣ�����Һ����ˮ�������c��H+��=1×10-12���ʴ�Ϊ��1×10-12��
���������⿼��������ʵĵ���ƽ�⡢����ˮ�⡢����Ũ�ȱȽϡ���ҺPHֵ���жϵȣ��漰����Ŀ�����ϴ����Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
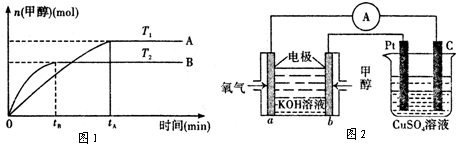
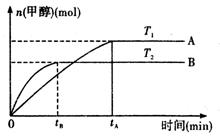
 CH3OH��g��+H2O ��g ��.�������������������£�ʵ�����¶ȶԷ�Ӧ��Ӱ������ͼ��ʾ��ע��T1��T2������300 �棩
CH3OH��g��+H2O ��g ��.�������������������£�ʵ�����¶ȶԷ�Ӧ��Ӱ������ͼ��ʾ��ע��T1��T2������300 �棩