��Ŀ����
5����ͼ1��ijú������ҵ����һ���֣���������ѧ֪ʶ������������⣺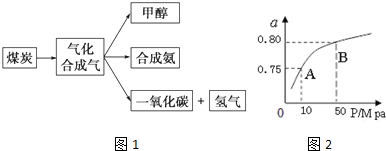
��1����֪�ò�ҵ����ij��Ӧ��ƽ�ⳣ������ʽΪK=$\frac{C��{H}_{2}����C��CO��}{C��{H}_{2}O��}$��������Ӧ��Ӧ�Ļ�ѧ����ʽ��C��s��+H2O��g��?CO��g��+H2��g����
��2���ϳɼ״�����Ҫ��Ӧ��2H2��g��+CO��g��?CH3OH��g����H=-90.8kJ•mol-1��t���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������п�ʼֻ����CO��H2����Ӧ10min���ø���ֵ�Ũ�����£�
| ���� | H2 | CO | CH3OH |
| Ũ�ȣ�mol•L-1�� | 0.2 | 0.1 | 0.4 |
�ڱȽϴ�ʱ�����淴Ӧ���ʵĴ�С��v����v�������������������=������
��ij�¶��·�ӦʱH2��ƽ��ת���ʣ�a������ϵ��ѹǿ��P���Ĺ�ϵ��ͼ1��ʾ����ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��=K��B�� �����������������=������
��3��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᣮͨ��״���£���amol/L��������bmol/LBa��OH��2��Һ�������ϣ���Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-�����ú�a��b�Ĵ���ʽ��ʾ�û����Һ������ĵ��볣��Ϊ$\frac{2b��1{0}^{-7}}{a-2b}$��
���� ��1������Ԫ���غ㡢ԭ���غ�֪����Ӧ���л���̼���ʲμӷ�Ӧ���ݴ���д���淴Ӧ����ʽ��
��2�����ȼ���״��ķ�Ӧ���ʣ��ٸ���ͬһʱ����ڸ����ʵķ�Ӧ����֮�ȵ����������֮�ȼ���������Ӧ���ʣ�
���ȼ���Ũ���̣��ٸ���Ũ������ƽ�ⳣ����Դ�С�жϷ�Ӧ���Ӷ�ȷ�����淴Ӧ������Դ�С��
�ۻ�ѧƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣻
��3��ͨ��״���£���a mol/L�Ĵ�����b mol/L Ba��OH��2��Һ�������ϣ���Һ������Ϊ���ᱵ��������������Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-��=bmol/L����Һ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ��������ƽ�ⳣ��K=$\frac{c��C{H}_{3}CO{O}^{-}����c��{H}^{+}��}{c��C{H}_{3}COOH��}$��
��� �⣺��1������Ԫ���غ㡢ԭ���غ�֪����Ӧ���л���̼���ʲμӷ�Ӧ���÷�Ӧ����ʽΪC��s��+H2O��g��?CO��g��+H2��g����
�ʴ�Ϊ��C��s��+H2O��g��?CO��g��+H2��g����
��2�����ɱ������ݿ�֪��l0min�ڼ״���Ũ�ȱ仯��Ϊ0.4mol/L����������Ũ�ȱ仯��Ϊ0.4mol/L��2=0.8mol/L����v��H2��=$\frac{0.8mol/L}{10min}$=0.08mol/��L•min����
�ʴ�Ϊ��0.08 mol•L-1•min-1��
���ɱ������ݿ�֪��10minʱ������Ũ��Ϊ0.2mol/L��CO��Ũ��Ϊ0.1mol/L���״���Ũ��Ϊ0.4mol/L�����ʱ��Ũ����Qc=$\frac{0.4}{0��{2}^{2}��0.1}$=100��С��ƽ�ⳣ��160���ʷ�Ӧ������Ӧ������У���V����V�����ʴ�Ϊ������
�ۻ�ѧƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣬�����¶���ͬ�����仯ѧƽ�ⳣ����ȣ��ʴ�Ϊ��=��
��3��ͨ��״���£���a mol/L�Ĵ�����b mol/L Ba��OH��2��Һ�������ϣ���Һ������Ϊ���ᱵ��������������Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-��=bmol/L����Һ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ��������ƽ�ⳣ��K=$\frac{c��C{H}_{3}CO{O}^{-}����c��{H}^{+}��}{c��C{H}_{3}COOH��}$=$\frac{b��1{0}^{-7}}{\frac{a}{2}-b}$=$\frac{2b��1{0}^{-7}}{a-2b}$��
�ʴ�Ϊ��$\frac{2b��1{0}^{-7}}{a-2b}$��
���� ���⿼�黯ѧƽ���йؼ��㡢������ʵĵ����֪ʶ�㣬���ؿ���ѧ����������������ע�⻯ѧƽ�ⳣ��ֻ���¶��йأ���ѹǿ������Ũ�ȶ��أ�ע�⣨3��������ʱ�����Һ�������������Ũ�Ƚ��ͣ�Ϊ�״��㣮

 53���ò�ϵ�д�
53���ò�ϵ�д�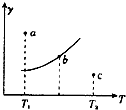 ��һ��Ӧ��2A+B?2C������A��B��C��Ϊ���壬��ͼ�������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�������������ȷ���ǣ�������
��һ��Ӧ��2A+B?2C������A��B��C��Ϊ���壬��ͼ�������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�������������ȷ���ǣ�������| A�� | �÷�Ӧ�Ƿ��ȷ�Ӧ | |
| B�� | b��ʱ��������ƽ��Ħ���������ٱ仯 | |
| C�� | T1�¶���a����Ҫ�ﵽƽ�⣬���Բ�ȡ����ѹǿ�ķ��� | |
| D�� | c��ʱv��������v���棩 |
| A�� | HCl | B�� | NaOH | C�� | ���� | D�� | CuSO4 |
| A�� |  | B�� |  | C�� |  | D�� |  |
 �������йظ÷��ӵ�������ȷ���ǣ�������
�������йظ÷��ӵ�������ȷ���ǣ�������| A�� | �÷��Ӻ�5���Ҽ���3���м� | |
| B�� | �÷����ǷǼ��Է��� | |
| C�� | �÷��ӵ�̼ԭ�Ӿ�����sp2�ӻ��������ɼ� | |
| D�� | ��������4��ԭ����ͬһֱ���� |
| A�� | NaNO2�Ⱦ����������־��л�ԭ�� | |
| B�� | 1 mol NaNO2��������������ȫ��Ӧ����NO��NO2��ת�Ƶ��ӵ����ʵ���Ϊ1 mol | |
| C�� | NaNO2��θ�����õ����ӷ���ʽΪ��2NO${\;}_{2}^{-}$+2H+=NO��+NO2��+H2O | |
| D�� | ʳ�á����ﶹѿ�����ܻ�����������ƶ������Σ�� |
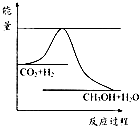 ��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й����������ı仯��
��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g������ͼ��ʾ�÷�Ӧ���й����������ı仯��