��Ŀ����
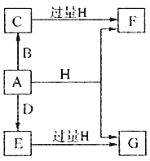
����Ŀ����п(ZnS)��һ����Ҫ�Ļ���ԭ�ϣ�������ˮ��������п�ķ���п����ȡ���乤����������ͼ��ʾ��
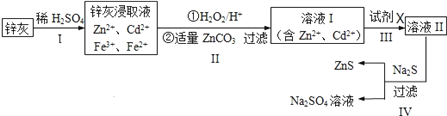
��1��Ϊ���п�ҵĽ�ȡ�ʣ��ɲ��õķ�����____________(�����)��
����ĥ �ڶ�ν�ȡ �������¶� �ܼ�ѹ �ݽ���
��2����������������е�������_______________(д��ѧʽ)��
��3��������пɵ�Cd���ʣ�Ϊ���������µ����ʣ��Լ�XӦΪ____________��
��4������������Ի���Na2SO4����ȡNa2S��
�ټ���ZnS�����Ƿ�ϴ�Ӹɾ��ķ�����_________________��
��Na2S���ɵ����ʵ�����Na2SO4��CH4�ڸ��¡�������������ȡ����ѧ��Ӧ����ʽΪ_______________________________��
��5�������������ZnCO3Ϊbmol�����������CdΪdmol,���õ�VL�����ʵ���Ũ��Ϊcmol/L��Na2SO4��Һ��������������п���к���пԪ�ص�����Ϊ____________��
���𰸡� �٢ڢۢ� Fe(OH)3��ZnCO3 Zn(��п) ȡ����ϴ��Һ�������Թܣ��μӼ���BaCl2��Һ�����ֻ�����δϴ������֮��ϴ�� Na2SO4+CH4![]() Na2S+2H2O+CO2 65(Vc-b-d)g
Na2S+2H2O+CO2 65(Vc-b-d)g
��������п�������ᷴӦ�ú��������ӡ�п���ӡ������ӡ��������ӵȵ���Һ������˫��ˮ����������������Ϊ���������ӣ���̼��п����pHʹFe(OH)3��ȫ���������˺�õ����������ӡ�п���ӵ���Һ��������Fe(OH)3��ZnCO3���������пɵ�Cd���ʣ�Ϊ���������µ����ʣ��Լ�XӦΪп�����˺����Һ��Ϊ����п��Һ������п��Һ�м������ƿɵ������ƺ���п��
��1�����衢�ʵ����¡���������ϸ�ɷ�ĩ�����衢��ν�ȡ�ȶ������п�ҵĽ�ȡ�ʣ������õķ����Ǣݼ�ѹ����ѡ�٢ڢۢ�����2����������ķ�����֪����������������ΪFe(OH)3��ZnCO3����3����������ķ�����֪���Լ�XӦΪп����4����ZnS�����Ǵ���������Һ�������ģ����Լ���ZnS�����Ƿ�ϴ�Ӹɾ��ķ�����ȡ���һ��ϴ��Һ�������Թܣ��μӼ���BaCl2��Һ�������ֻ�����δϴ������֮����ϴ�����ڵ����ʵ�����Na2SO4��CH4�ڸ��¡���������������Na2S������Ԫ���غ��֪���û�ѧ��Ӧ����ʽΪ��Na2SO4+CH4![]() Na2S+2H2O+CO2����5������������CdΪdmol���������û��ӵ�п�����ʵ���Ϊdmol�������Ƶ����ʵ���Ϊ��VL��cmol/L=cVmol����������п�����ʵ���ΪcVmol������пԪ���غ��֪����Ʒ��пԪ�ص����ʵ���Ϊ��cVmol-dmol-bmol������п���к���пԪ�ص�����Ϊ��65g/mol��(cVmol-dmol-bmol)=65(Vc-b-d)g��
Na2S+2H2O+CO2����5������������CdΪdmol���������û��ӵ�п�����ʵ���Ϊdmol�������Ƶ����ʵ���Ϊ��VL��cmol/L=cVmol����������п�����ʵ���ΪcVmol������пԪ���غ��֪����Ʒ��пԪ�ص����ʵ���Ϊ��cVmol-dmol-bmol������п���к���пԪ�ص�����Ϊ��65g/mol��(cVmol-dmol-bmol)=65(Vc-b-d)g��

����Ŀ��������AlP��ͨ������Ϊһ�ֹ�����Ѭ��ɱ�������ˮ������������߶���PH3���壨�۵�Ϊ-132������ԭ��ǿ����������ȫ���涨������ʳ�������PH3�ƣ��ĺ���������0.05mg��kg-1ʱ����ʳ�����ϸ�֮����ʳ�������ϸ�ij��ѧ��ȤС���ͬѧͨ�����з�������ʳ�в��������ﺬ���������о���
���������̡�
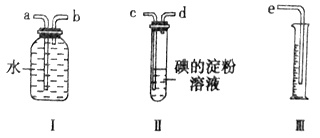
��װ����װ����PH3�IJ�����������ת��KMnO4������Һ���������Ʊ���Һ�ζ���
��ʵ��װ�á�
��֪ C ��ʢ�� 100 gԭ����E ��ʢ�� 20.00 mL 1.13��10-3 mol L-1KMnO4��Һ��H2SO4�ữ)����ش��������⣺

��1������D��������____________________________��
��2��B��ʢ�н���ûʳ����ļ�����Һ�������������տ����е�O2����ֹ����װ��C�����ɵ�PH3����A��ʢװKMnO4��Һ��������_________________________��
��3����֪MnO4-����ԭΪMn2+����0.1mol PH3ǡ�ñ�0.16mol KMnO4���գ���PH3�������IJ�����__________��д��E�з�����Ӧ�����ӷ���ʽ��_________________________
��4���ռ�E������Һ����ˮϡ����250mL��ȡ25.00mL����ƿ�У���5.0��10-4mol L-1��Na2SO3����Һ�ζ�ʣ���KMnO4��Һ��
����ʵ�����ģ��� | 1 | 2 | 3 |
mL | 11.02 | 11.30 | 10.98 |
���ζ��ﵽ�յ�������ǣ�________________________________��
�����ݴ���������Na2SO3����Һ____mL�����ԭ���������PH3�ƣ��ĺ���Ϊ______________________mg kg-1��
����C�з�Ӧ��ȫ������ͨ����������У�4���еĵζ�������������Na2SO3����Һ�����____________��ѡ����ƫ��������ƫС��������������