��Ŀ����
��ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ����20��������������Ϊδ���Ķ���ȼ������Դ���о������Ѹ�ٷ�չ��
��1����֪��2H2(g)+ O2(g)==2H2O(g)����H=-483.6kJ/mol�� H2O(g)==H2O(l)����H=-44kJ/mol��
��д��H2ȼ���ȵ��Ȼ�ѧ����ʽ��
____________________________________________________________��
��2��ijѧ�����Ĵ������ϣ�������С�22������ˮ��ȡ�������о����� �����о������У�����Ϊ����ȷ��ѡ����_________________��
���о���ˮ��������ѧ��Ӧ���������ȡ������ͬʱ�ͷ�����
���跨��̫����۽����������£�ʹˮ�ֽ��������
��Ѱ�Ҹ�Ч�������������ˮ��һ�������·ֽ����������ת����
��Ѱ�����⻯ѧ���ʣ����ڿ���������Դ���Էֽ�ˮ��ȡ����
��3��������Ϊ��Դؽ���������һ������������ϵĿ������о�����ijЩ���ɽ���������ԭ���γ��⻯���ԭ������ڽ����ľ����϶֮�䣬����ɲ��̶���ͨ���Ƿǻ�ѧ�����ģ���LaH2.76������֪��״���£�1������ٷ۴�Լ������896������������ٷ۵��ܶ�Ϊ10.64g/cm3������д���٣�Pd�����⻯��Ļ�ѧʽ___________________��
��1��H2(g)+1/2 O2(g)==H2O(l)����H=-285.8.kJ/mol��
��2���� ��
��3��PdH0.8����д��Pd5H4Ҳ�ɣ�

 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�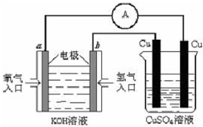
 ��ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ��Ϊ����CO�Դ�������Ⱦ��ij�о���ѧϰС�����о�CO��H2O��Ӧת��Ϊ��ɫ��ԴH2��
��ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ��Ϊ����CO�Դ�������Ⱦ��ij�о���ѧϰС�����о�CO��H2O��Ӧת��Ϊ��ɫ��ԴH2��
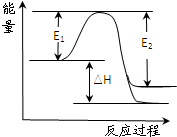
 CH3OH(g)����H ����90.8 kJ��mol��1
CH3OH(g)����H ����90.8 kJ��mol��1