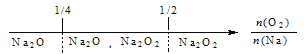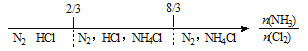��Ŀ����
����Ŀ�����������������������ǵ�����������ʡ�
(1)SO2�ж�,�����γ�����,�Ǵ�����Ҫ��Ⱦ��֮һ��ʯ��-ʯ�෨�ͼ�dz��õ���������ʯ��-ʯ�෨������ԭ��:
��SO2+Ca(OH)2=CaSO3��+H2O��2CaSO3+O2+4H2O=2(CaSO4��2H2O)�������ԭ��:����SO2��β��ͨ���������ռ���Һ��,��д����Ӧ�Ļ�ѧ��Ӧ����ʽ__________;
��֪:
�Լ� | Ca(OH)2 | NaOH |
�۸�(Ԫ/kg) | 0.36 | 2.9 |
��ʯ��-ʯ�෨���,����ŵ������տ졢Ч�ʸ�,ȱ����__________;
(2)ʯ��-ʯ�෨�ͼ�Ļ�����,�����˫�,��ʵ������ѭ�����á�

����������,ʵ��ѭ�����õ�������__________,���û�ѧ����ʽ��ʾ��Na2SO3��Һ�м���CaO��ķ�Ӧԭ��__________��
(3)һ�������°��������������������ת��Ϊ����Ⱦ�����ʡ�д�������Ͷ���������һ�������·�Ӧ�Ļ�ѧ����ʽ��____________
(4)������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�
NO2��NO��2NaOH��2NaNO2��H2O
2NO2��2NaOH��NaNO2��NaNO3��H2O
����VLijNaOH��Һ����ȫ����n mol NO2��m mol NO��ɵĴ�����Ⱦ�
�������ռ���Һ�����ʵ���Ũ������Ϊ________mol��L��1��
����������Һ��c(NO3-)��c(NO2-)��1��9����ԭ���������NO2��NO�����ʵ���֮��n��m��________��
���𰸡�SO2+2NaOH=Na2SO3+H2O �ɱ��ϸ� NaOH CaO+H2O=Ca(OH)2 Ca(OH)2+Na2SO3=CaSO3��+2NaOH 6NO2��8NH3![]() 7N2��12H2O (m+n)/V 3��2
7N2��12H2O (m+n)/V 3��2
��������
��1�����ݶ���������������Ʒ�Ӧ����������д�����ݱ������ݷ����жϣ�
��2����������ת���ص�����ʵ����ʷ����жϣ�
��3�������ɽ���������ת��Ϊ����Ⱦ�����ʣ�������Ӧ���ǵ�����ˮ���ݴ���д��
��4���ٸ���������������ƵĹ�ϵ���㣻
�ڸ��ݶ���������һ���������������Ʒ�Ӧ�ķ���ʽ�������
��1������SO2��β��ͨ���������ռ���Һ�������������ƺ�ˮ����Ӧ�Ļ�ѧ��Ӧ����ʽΪSO2+2NaOH��Na2SO3+H2O�����ݱ������ݿ�֪�������Ƶijɱ��ߣ����ʯ��-ʯ�෨�����ȱ���dzɱ��ϸߣ�
��2����������ͼ��֪����������ˮ�����������ƣ��������ƺ��������Ʒ�Ӧ����������ƺ��������ƣ�����������Һ�����ն���������ʵ��ѭ�����õ�������NaOH����������������֪��Na2SO3��Һ�м���CaO��ķ�Ӧԭ��ΪCaO+H2O��Ca(OH)2��Ca(OH)2+Na2SO3��CaSO3��+2NaOH��
��3��һ�������°��������������������ת��Ϊ����Ⱦ�����ʣ��������ǵ�����ˮ�������Ͷ���������һ�������·�Ӧ�Ļ�ѧ����ʽΪ6NO2��8NH3![]() 7N2��12H2O��
7N2��12H2O��
��4���ٸ��ݷ���ʽNO2��NO��2NaOH��2NaNO2��H2O��2NO2��2NaOH��NaNO2��NaNO3��H2O��ֻ֪Ҫ��NO����������ԭ�Ӻ��������ƵĹ�ϵ��1:1������ȫ����n mol NO2��m mol NO�����ռ���Һ�����ʵ���Ũ������Ϊ(m+n)/V mol��L��1��
�ڸ��ݷ���ʽ��֪
NO2��NO��2NaOH��2NaNO2��H2O
m m 2m
2NO2��2NaOH��NaNO2��NaNO3��H2O
(n��m) (n��m)/2 (n��m)/2
��������Һ��c(NO3-):c(NO2-)��1:9����(n��m)/2:(n+3m)/2��1:9�����n:m��3:2����ԭ���������NO2��NO�����ʵ���֮��Ϊ3:2��

����Ŀ�����Ȼ���������ýȾ����������ͼ��ʾװ�ÿ����Ʊ����Ȼ��������ּг�װ������ȥ����

�й���Ϣ���±���
��ѧʽ | SnCl2 | SnCl4 |
�۵�/�� | 246 | -33 |
�е�/�� | 652 | 144 |
�������� | ��ɫ���壬������ | ��ɫҺ�壬��ˮ�� |
�ش��������⣺
��1����װ���������ܵĽ�ˮ��Ϊ___________���a����b������
��2���ü�װ����������MnO4-����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ________________________��
��3����װ����ͼ���Ӻã���������ԣ���������Ũ���ᣬ���۲쵽___________��������ʼ���ȶ�װ�ã����ۻ����ʵ����������������������ȶ�װ�ã���ʱ�������ȶ�װ�õ�Ŀ���ǣ��ٴٽ�����������Ӧ����_______________________________��
��4�����ȱ����װ�ã����ܲ����ĺ����___________________����װ�õ�������__________________��
��5��ijͬѧ��Ϊ��װ���еķ�Ӧ���ܲ���SnCl2���ʣ������Լ��п����ڼ���Ƿ����SnCl2 ����_______________�����ţ���
a.FeCl3��Һ������KSCN�� b.H2O2��Һ C.��ˮ d.AgNO3��Һ
��6����Ӧ����ȥ����1.19g����Ӧ������װ�õ��Թ����ռ���2.38 gSnCl4����SnCl4�IJ���Ϊ____________________��