��Ŀ����
����Ŀ��������H��He��N��Na��Mg��Si��Ԫ����������δ������Դ������
��1��3He�Ǹ�Ч����ԭ������ԭ�Ӻ���������Ϊ__________��
��2��Na��ԭ�ӽṹʾ��ͼΪ_________��Na����������ȫȼ�����ò���ĵ���ʽΪ________��
��3��MgCl2�ڹ�ҵ��Ӧ�ù㷺������MgO�Ʊ���
��MgO���۵��BaO���۵�________(����������������)��
��������ij��ʯ�������õ���MgO�к�������SiO2����ȥSiO2�����ӷ���ʽΪ��_________��
��MgO��̿�ۺ�������һ�������·�Ӧ���Ʊ�MgCl2����β����������NaOH��Һ��ȫ���գ� �����ɵ���Ϊ__________(д��ѧʽ)��
��4�������к��з�����3He������������1kg3He��ͬʱ�ɵ�6000kgH2��700 kg N2����������H2��N2Ϊԭ�Ͼ�һϵ�з�Ӧ���������������________kg��
���𰸡���1��1
��2��![]() ��
��![]()
��3������ ��2OH����SiO2��SiO32����H2O��ԭ�Ӿ��� ��NaCl��NaClO��Na2CO3
��4��3950
��������
�����������1��3He�Ǹ�Ч��ԭ�ϣ���ԭ�Ӻ��ڵ�������=������-������=3-2=1��
��2������11��Ԫ�أ�ԭ�����������Ϊ11�����ݺ�������Ų�����д��ԭ�ӽṹʾ��ͼ![]() ������������ȼ�����ɹ������ƣ��������������ӻ��������ɹ��������Ӻ������ӹ��ɣ�������ʽΪ��
������������ȼ�����ɹ������ƣ��������������ӻ��������ɹ��������Ӻ������ӹ��ɣ�������ʽΪ��![]() ��
��
��3��������þ�������������ӻ���������Ӱ뾶����þ���Ӱ뾶������þ�γɵ�������ǿ���۵����
������þ�Ǽ����������������������������������ö���������������������Һ��ȥ����Ӧ�����ӷ���ʽΪ��SiO2+2OH-=SiO32-+H2O�����������ǹ�ԭ�Ӻ���ԭ�ӹ��ɣ�ÿ����ԭ�Ӻ��ĸ���ԭ���γɹ��ۼ���ÿ����ԭ�Ӻ�������ԭ���γɹ��ۼ�������ԭ�Ӿ�����
��MgO��̼�ۺ�������һ�������·�Ӧ���Ʊ�MgCl2����Ӧ����Ϊ������̼����β����������NaOH��Һ��ȫ��������������������̼���������Ʒ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O��Cl2+2NaOH=NaCl+NaClO+H2O������ΪNaCl��NaClO��Na2CO3��
��4����6000kg H2��700kg N2�����ݻ�ѧ����ʽN2+3H2=2NH3�����Եõ�H2��N2Ϊԭ�����֪�������������ݵ����е�Ԫ���غ���㣬��һϵ�з�Ӧ��������̼����淋�����=700kg��28g/mol��2��79g/mol=3950kg��

����Ŀ��������X����(�����)Y��Һ�У������ɳ��������ʵ��������X�����ʵ�����ϵ��ͼ��ʾ������ͼʾ�������( )
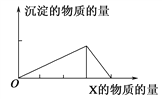
A | B | C | D | |
X | NaOH | HCl | CO2 | AlCl3 |
Y | AlCl3 | NaAlO2 | Ca(OH)2 | NaOH |
A. A B. B C. C D. D




