��Ŀ����
����Ŀ����A��B��C��D���ֶ�����Ԫ�أ�AԪ���γɵ�-2�������ӱȺ�ԭ�ӵĺ����������8����BԪ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʣ�CΪԭ�Ӻ�����12�����ӵĶ��۽�������2.4gC��������ˮ��Ӧʱ���ڱ�״���·ų�����2.24L��D��M����7�����ӡ�
��1��д��A�����ӽṹʾ��ͼ��___________��B�����ڱ���λ�ã�______________��д��C��Ԫ�ط���________��D����������ﻯѧʽ_______��
��2���Ƚ�D����̬�⻯����H2S��HF���ȶ��ԣ�______>_______>______
��3��C��D��Ԫ������������ˮ�������ѧ��Ӧ�����ӷ���ʽ______________________
���𰸡�![]() ��3���ڵ�IA�� Mg Cl2O7 HF>HCl>H2S Mg(OH)2+2H+==Mg2++2H2O
��3���ڵ�IA�� Mg Cl2O7 HF>HCl>H2S Mg(OH)2+2H+==Mg2++2H2O
��������
A��B��C��D���ֶ�����Ԫ�أ�AԪ���γɵ�-2�������ӱȺ�ԭ�ӵĺ����������8����A��O��BԪ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʣ�B��Na�����ɵ�������0.1mol����C�����ԭ��������2.4��0.1��24��CΪԭ�Ӻ�����12�����ӵĶ��۽�������C����Mg��D��M����7�����ӣ�D��Cl��
��1���������ӽṹʾ��ͼΪ![]() ���������ڱ���λ��Ϊ��3���ڵ�IA�壬C��Ԫ�ط�����Mg��Cl����������ﻯѧʽΪCl2O7����2���ǽ�������F��Cl��S����D����̬�⻯����H2S��HF���ȶ���ΪHF��HCl��H2S����3��C��D��Ԫ������������ˮ�������ѧ��Ӧ�����ӷ���ʽΪMg(OH)2+2H+��Mg2++2H2O��
���������ڱ���λ��Ϊ��3���ڵ�IA�壬C��Ԫ�ط�����Mg��Cl����������ﻯѧʽΪCl2O7����2���ǽ�������F��Cl��S����D����̬�⻯����H2S��HF���ȶ���ΪHF��HCl��H2S����3��C��D��Ԫ������������ˮ�������ѧ��Ӧ�����ӷ���ʽΪMg(OH)2+2H+��Mg2++2H2O��

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�����Ŀ��ij�о���ѧϰС�����о�Ӱ��п��ϡ���ᷴӦ���ʵ�����������±�����ʵ����Ƶ��й����ݣ�
ʵ�� ��� | п������/g | п��״̬ | c(H2SO4) /mol��L��1 | V(H2SO4) /mL | ��Ӧǰ�� Һ���¶�/�� | ���Ӽ� |
1 | 0.65 | ��״ | 0.5 | 50 | 20 | �� |
2 | 0.65 | ��ĩ | 0.5 | 50 | 20 | �� |
3 | 0.65 | ��״ | 0.5 | 50 | 20 | 2��CuSO4��Һ |
4 | 0.65 | ��ĩ | 0.8 | 50 | 20 | �� |
5 | 0.65 | ��ĩ | 0.8 | 50 | 35 | 2��CuSO4��Һ |
��1���ڴ�5��ʵ���У��ж�п��ϡ���ᷴӦ���ʴ�С����ķ�����ͨ�������ⶨ______________________�����жϣ�����������ʵ����________(��ʵ�����)��
��2����п��ϡ���ᷴӦ��ʵ��1��2������________�Է�Ӧ������Ӱ�죻ʵ��1��3������________�Է�Ӧ������Ӱ�졣
��3������ʵ��2ʱ��С��ͬѧ����ʵ����̻��Ƶı�״���µ��������V��ʱ�� t��ͼ������ͼ��ʾ��
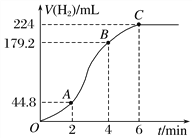
����OA��AB��BC�����з�Ӧ����������________��ԭ����______________________��
��2��4 min���������Ũ�ȱ仯��ʾ�ķ�Ӧ����(������Һ���������)Ϊ________________________________________________________________________��
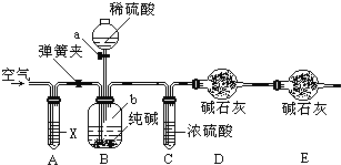
��4��������ͼ2װ����֤�ǽ����ԣ�C��Si��B�м�Na2CO3��C�м�Na2SiO3��Һ��A��Ӧ�ü���__________________________��C�з�Ӧ�Ļ�ѧ����ʽ��________________________��Dװ�õ�������_______________________________________��
����Ŀ��Ϊģ�⺣ˮ�Ʊ� MgO��ijͬѧ��Ʋ����������ʵ�飺
ģ�⺣ˮ������c(mol/L) | Na�� | Mg2�� | Ca2�� | Cl�D | HCO3 |
0.439 | 0.050 | 0.011 | 0.560 | 0.001 |
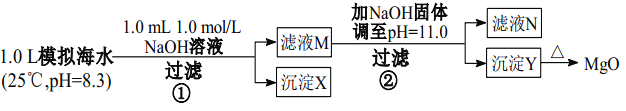
����˵����ȷ����
A. ģ�⺣ˮ�ʼ��ԣ���˵�� HCO3-�ĵ�����������ˮ������
B. ������ X Ϊ CaCO3�������� Y Ϊ Mg(OH)2
C. MgCO3���ܽ�ȱ� Mg(OH)2 ��С
D. ��Һ M �д��� Mg2���������� Ca2��
����Ŀ����ѧ�������о���������CH4��CO2��ת�������á���ش��������⣺
��1������һ���ռ��˶�״̬�ĵ�����ԭ�Ӻ�����ֵĸ����ܶȷֲ�����____________�����������ڻ�̬14Cԭ���У��������___________�������෴�ĵ��ӡ�
��2��CH4��CO2����������Ԫ�ص縺�Դ�С�����˳��Ϊ__________________________��
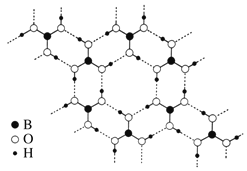
��3��һ�������£�CH4��CO2������H2O�γ���״�ṹ(����ͼ��ʾ)��ˮ���ᄃ�壬����ز������±���CH4��H2O�γɵ�ˮ�����׳�����ȼ������
���� ���ӡ����� | ����ֱ��/nm | ������H2O�Ľ����E/kJ��mol��1 |
CH4 | 0.436 | 16.40 |
CO2 | 0.512 | 29.91 |
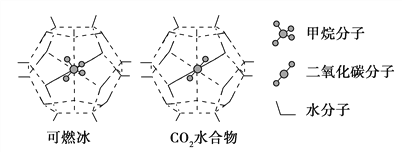
�����й���CH4��CO2��˵����ȷ����________(�����)��
a��CO2�������2��������2������
b��CH4�����к��м��Թ��ۼ����Ǽ��Է���
c����Ϊ̼�������С��̼����������CH4�۵����CO2
d��CH4��CO2������̼ԭ�ӵ��ӻ����ͷֱ���sp3��sp
��Ϊ�������������ȼ�������п�ѧ�������CO2�û�CH4�����롣��֪��ͼ����״�ṹ�Ŀ�ǻֱ��Ϊ0. 586 nm����������ͼ�����ṩ�����ݷ���������������������______________________________________��