��Ŀ����
(14��)

 �±���Ԫ�����ڱ���һ���֣���ش��й����⣺
�±���Ԫ�����ڱ���һ���֣���ش��й����⣺
 ��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ ��
��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ ��
 ��2���������γ��������������Ԫ���� (��Ԫ�ط��ű�ʾ)��д����Ԫ�ص�
��2���������γ��������������Ԫ���� (��Ԫ�ط��ű�ʾ)��д����Ԫ�ص� ���������������ˮ���ﷴӦ�Ļ�ѧ����ʽ
���������������ˮ���ﷴӦ�Ļ�ѧ����ʽ
 ��3����Ԫ�����Ԫ���γɻ�����ĵ���ʽ
��3����Ԫ�����Ԫ���γɻ�����ĵ���ʽ
 ��4���١��ڡ��ޡ�������Ԫ�ص�����������ˮ������������ǿ����
��4���١��ڡ��ޡ�������Ԫ�ص�����������ˮ������������ǿ����
 (�ѧʽ)��
(�ѧʽ)��
 ��5����Ԫ�����Ԫ�����ߺ˵����֮���� ��
��5����Ԫ�����Ԫ�����ߺ˵����֮���� ��
 ��6�����ʵ�鷽�����ȽϢ���ⵥ�������Ե�ǿ�����뽫���������±���
��6�����ʵ�鷽�����ȽϢ���ⵥ�������Ե�ǿ�����뽫���������±���



 �±���Ԫ�����ڱ���һ���֣���ش��й����⣺
�±���Ԫ�����ڱ���һ���֣���ش��й����⣺ ���� ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 2 | | | | �� | �� | | �� | |
| 3 | | | �� | | | �� | �� | �� |
| 4 | �� | �� | | | | | �� | |
 ��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ ��
��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ �� ��2���������γ��������������Ԫ���� (��Ԫ�ط��ű�ʾ)��д����Ԫ�ص�
��2���������γ��������������Ԫ���� (��Ԫ�ط��ű�ʾ)��д����Ԫ�ص� ���������������ˮ���ﷴӦ�Ļ�ѧ����ʽ
���������������ˮ���ﷴӦ�Ļ�ѧ����ʽ  ��3����Ԫ�����Ԫ���γɻ�����ĵ���ʽ
��3����Ԫ�����Ԫ���γɻ�����ĵ���ʽ  ��4���١��ڡ��ޡ�������Ԫ�ص�����������ˮ������������ǿ����
��4���١��ڡ��ޡ�������Ԫ�ص�����������ˮ������������ǿ����  (�ѧʽ)��
(�ѧʽ)�� ��5����Ԫ�����Ԫ�����ߺ˵����֮���� ��
��5����Ԫ�����Ԫ�����ߺ˵����֮���� �� ��6�����ʵ�鷽�����ȽϢ���ⵥ�������Ե�ǿ�����뽫���������±���
��6�����ʵ�鷽�����ȽϢ���ⵥ�������Ե�ǿ�����뽫���������±���


| ʵ�鲽�� | ʵ����������� |
| |       |
��1���� ��2��Al 2Al + 2KOH + 2H2O = 2KAlO2 + 3H2 ��
��3���� ��4��HClO4 ��5��26
��6��������������Ҳ���֣�
��3���� ��4��HClO4 ��5��26
��6��������������Ҳ���֣�
| ʵ�鲽�� | ʵ����������� |
| ��һ֧С�Թ��м�������NaBr��Һ���μ���ˮ | �����Һ����ɫ��Ϊ��ɫ��˵��Cl2�ķǽ����Ա�Br2ǿ |
��

��ϰ��ϵ�д�
�����Ŀ
 ��Ӧ�Ļ�ѧ����ʽΪ
��Ӧ�Ļ�ѧ����ʽΪ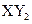 �ͻ������X��Y ��ԭ������֮�����Ϊ2��5
�ͻ������X��Y ��ԭ������֮�����Ϊ2��5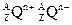 ��ʾ�����й��ڸ�����������ȷ���ǣ� ��
��ʾ�����й��ڸ�����������ȷ���ǣ� ��