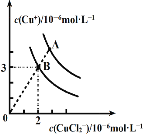
��Ŀ����
����Ŀ����ͼ�Dz��ֶ�����Ԫ�صij������ϼ���ԭ�������Ĺ�ϵ��
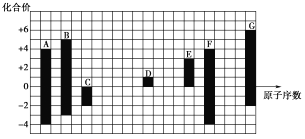
(1)Ԫ��Aλ�����ڱ��е�______����______�壻
(2)Ԫ��G������������Ӧˮ�����Ũ��Һ�����ȵ�A�ĵ��ʷ�Ӧ_________��
(3)D��G���γɻ�����������ѧ������Ϊ______��
(4)C2����D����G2���뾶�ɴ�С��˳����________(�����ӷ���)��
(5)F��C���γɵĻ�������___________���塣
(6)C��D�γɵľ���ǿ�����ԵĻ�����ĵ���ʽΪ________��
���𰸡��� ��A C+2H2SO4 ��Ũ��![]() CO2��+2SO2��+2H2O ���Ӽ� S2��>O2��>Na�� ԭ��
CO2��+2SO2��+2H2O ���Ӽ� S2��>O2��>Na�� ԭ�� ![]()
��������
A��B��C��D��E��F��G��Ϊ������Ԫ�أ���ԭ������������A��F������ϼ۾�Ϊ+4�ۣ���AΪ̼Ԫ�أ�FΪ��Ԫ�أ�C��G����ͻ��ϼ۾�Ϊ-2�ۣ���CΪ��Ԫ�أ�GΪ��Ԫ�أ�B������ϼ�Ϊ+5�ۣ�ԭ������С������Ϊ��Ԫ�أ�D�Ļ��ϼ�Ϊ+1�ۣ�ԭ����������������DΪ��Ԫ�أ�E�Ļ��ϼ�Ϊ+3�ۣ�Ϊ��Ԫ�أ��ݴ˷�����
�������Ϸ�����A��B��C��D��E��F��G�ֱ�ΪC��N��O��Na��Al��Si��S����
(1)̼Ϊ6��Ԫ�أ�����������Ϊ4��λ�����ڱ��еڶ����ڢ�A �壬
�ʴ�Ϊ��������A��
(2)��Ԫ�ص�����������Ӧˮ����ΪH2SO4��Ũ���������ȵ�C����������ԭ��Ӧ���ɶ�����������̼��ˮ����ѧ����ʽΪ��C+2H2SO4 ��Ũ��![]() CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4 ��Ũ��![]() CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O��
(3)DΪNa��Ϊ���ý�����GΪS��Ϊ���÷ǽ���������������ʽ������Ϊ���ӻ����������ѧ������Ϊ���Ӽ���
�ʴ�Ϊ�����Ӽ���
(4)��������Ų���ͬ�����ӣ��˵����Խ��뾶ԽС���뾶O2��>Na�������Ӳ���Խ��뾶Խ�뾶S2��>O2�������뾶�ɴ�С��˳����S2��>O2��>Na����
�ʴ�Ϊ��S2��>O2��>Na����
(5)FΪSi��CΪO��Si��O�γ�SiO2��ԭ�Ӿ��壬
�ʴ�Ϊ��ԭ�ӣ�
(6)CΪO��DΪNa��O��Na�γɵľ���ǿ�����ԵĻ�����ΪNa2O2�������ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�