��Ŀ����

��1����ij�ݻ�Ϊ10L���ܱ������У��п��淴Ӧ��
mA��g��+nB��g��?pC��g��+qD��s����H��0��
��ͼ1Ϊij��Ӧ�����и��������ʵ���n��mol����ʱ��t��min���ı仯����ͼ��
����0��15min�ڵ�ƽ����Ӧ���ʣ�v��B��=______��
�ڸ÷�Ӧ��ƽ�ⳣ������ʽΪ______
��������Щ���������ٱ仯ʱ���Ա����÷�Ӧ�Ѿ�����ƽ��״̬______������ĸ����ѡ����ѡ���۷֣�
a����������ѹǿ b�����������ܶ�
c��������������� d��c��A����c��B���ı�ֵ
��2�����÷�Ӧ������Ӧ������ʱ��Ĺ�ϵ��ͼ2��ʾ���������������������£�t2ʱ�ı������������______��
��1�������£�0.1mol?L-1��CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����______������ţ�
A��c��H+�� B��c��H+��/c��CH3COOH��
C��c��H+��?c��OH-�� D��c��OH-��/c��H+��
��2�������Ϊ100mLpH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ3��ʾ����Ka��HX��______ ������ڡ�����С�ڡ����ڡ���Ka��CH3COOH����
��3��23��ʱ����CH3COOH��CH3COONa�Ļ����Һ�У������pH=6������Һ������Ũ�ȴ�С��ϵ��______��c��CH3COO-��/c��CH3COOH��=______�� ����֪����ĵ���ƽ�ⳣ��Ka=1.8×10-5��
�ٸ���v=
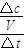 ����v��B����
����v��B�����ڻ�ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ���ݴ���д��
�ۻ�ѧ��Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��ٷ����ı䣬�ɴ�������һЩ���������䣬�Դ˽����жϣ�ע��ѡ���жϵ�������Ӧ�淴Ӧ���з����仯�����������ɱ仯�����䣬˵������ƽ�⣻
��2����ͼ��֪���ı�������˲������Ӧ���ʽ��ͣ��������Ӧ���ʼ������ͣ�˵���ı�����ƽ��������Ӧ�����ƶ�����Ϸ�Ӧ�������з������
��1��A.0.1mol?L-1��CH3COOH��Һ��ˮϡ�ͣ���Һ���Լ�����
B������ĵ���̶�������Һ��n��H+������n��CH3COOH����С��Ũ��֮�ȵ������ʵ���֮�ȣ�
C��һ���¶���c��H+��?c��OH-��Ϊ��ֵ��
D.0.1mol?L-1��CH3COOH��Һ��ˮϡ�ͣ���Һ���Լ�����c��H+����С��c��OH-������
��2����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
��3��25��ʱ�����ҺpH=6����Һ�����ԣ�˵������ĵ���̶ȴ��ڴ������ˮ��̶ȣ��ݴ��ж�����Ũ�ȴ�С˳��
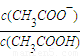 =
=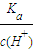 ���ݴ˼��㣮
���ݴ˼��㣮����⣺��1����ͼ��֪��0��15min�ڡ�n��A��=0.5mol����n��B��=3mol-1.5mol=1.5mol����n��C��=1mol����m��n��p=0.5mol��1.5mol��1mol=1��3��2����ӦΪA��g��+3B��g��?2C��g����
��v��B��=
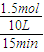 =0.01mol/��L?min�����ʴ�Ϊ��0.01mol/��L?min����
=0.01mol/��L?min�����ʴ�Ϊ��0.01mol/��L?min������A��g��+3B��g��?2C��g����ƽ�ⳣ��k=
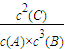 ���ʴ�Ϊ��
���ʴ�Ϊ��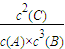 ��
����a������Ӧ��������ʵ�����С���淴Ӧ����ѹǿ��С����������ѹǿ���䣬˵������ƽ�⣬��a��ȷ��
b�������������������䣬�������ݻ����䣬���������ܶ�ʼ�ղ��䣬��b����
c�����������һ�����������������ʼ�յ����������ݻ�����c����
d��c��A����c��B���ı�ֵ����ʼͶ�����йأ�������ѧ������֮��Ͷ�룬����֮��Ϊ��ֵ���ڻ�ѧ������֮�ȣ�����˵������ƽ��״̬����d����
�ʴ�Ϊ��a��
��2����ͼ��֪���ı�������˲������Ӧ���ʽ��ͣ��������Ӧ���ʼ������ͣ�˵���ı�����ƽ��������Ӧ�����ƶ�����ӦA��g��+3B��g��?2C��g��������Ӧ��������ʵ�����С�ķ��ȷ�Ӧ��t2ʱ�ı������������Ϊ����ѹǿ������Ϊ�����¶ȣ��ʴ�Ϊ�������¶ȣ�
��1��A.0.1mol?L-1��CH3COOH��Һ��ˮϡ�ͣ���Һ���Լ�����c��H+�����ͣ���A����
B������ĵ���̶�������Һ��n��H+������n��CH3COOH����С��Ũ��֮�ȵ������ʵ���֮�ȣ���
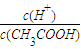 ����B��ȷ��
����B��ȷ��C��һ���¶���c��H+��?c��OH-��Ϊ��ֵ����C����
D.0.1mol?L-1��CH3COOH��Һ��ˮϡ�ͣ���Һ���Լ�����c��H+����С��c��OH-������
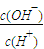 ����D��ȷ��
����D��ȷ���ʴ�Ϊ��BD��
��2����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ��HX�ĵ���ƽ�ⳣ������Ka��HX������Ka��CH3COOH�����ʴ�Ϊ�����ڣ�
��3��25��ʱ�����ҺpH=6����Һ�����ԣ�c��H+����c��OH-����˵������ĵ���̶ȴ��ڴ������ˮ��̶ȣ���c��CH3COO-����c��Na+��������ĵ���̶Ȳ�������Ũ�ȴ�С˳��c��CH3COO-����c��Na+����c��H+����c��OH-����
25��ʱ�����ҺpH=6��c��H+��=1.0×10-6mol/L��
 =
=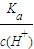 =
=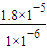 =18��
=18���ʴ�Ϊ��c��CH3COO-����c��Na+����c��H+����c��OH-����18��
�����������ۺ��Խϴ�Ϊƴ������Ŀ���漰��ѧƽ��ͼ��ѧ��Ӧ���ʡ���ѧƽ�ⳣ����Ӱ��ƽ������ء�����ˮ�⣬������ʵĵ���ȣ���Ŀ�Ѷ��еȣ�ע�����л�ѧƽ�ⳣ����д���岻��Ҫд����������Ӱ��������ֵĻ�ѧ��������

�����ǶԻ�ѧ��Ӧ�仯���̼�������о�����Ҫ��ش����⣺
��.���ڷ�Ӧ���ʺ��ȵ��о�
��1����֪25��ʱ�й�����ĵ���ƽ�ⳣ����
���ữѧʽ | CH3COOH | HCN | H2CO3 |
����ƽ�ⳣ��(25��) | 1.8��10��5 | 4.9��10��10 | K1��4.3��10��7 K2��5.6��10��11 |
������ʵ���Ũ�ȵ���CH3COONa����NaCN����Na2CO3����NaHCO3��Һ��pH�ɴ�С��˳��Ϊ__________(����)��
��2����֪2SO2(g)��O2(g)??2SO3(g)����H����196.6 kJ��mol��1����һ���ݻ�Ϊ2 L�������м���2 mol SO2��1 mol O2����ij�¶��³�ַ�Ӧ������30 min�ﵽƽ�⣬�ų�����176.94 kJ�������SO2��ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v(SO2)��________��
��3����ͼΪij�¶��£�CuS(s)��ZnS(s)��FeS(s)�ֱ�����Һ�дﵽ�����ܽ�ƽ�����Һ��S2��Ũ�ȡ�����������Ũ�ȱ仯�������������ֳ����м����ᣬ�����ܽ����________��

�������ɵ�ZnS��Һ�е�����������ͬŨ�ȵ�Cu2����Fe2������Һ����ZnS������ת��Ϊ________(�ѧʽ)������
��.���ڵ绯ѧ���о�
ȫ��Һ�������һ�����͵���ɫ�������ܵ�ء������ܷ�ӦΪ��VO2+��2H����V2�� V3����VO2����H2O������ʱ������ӦʽΪ__________________________���ô˵�ص��1 L 1 mol��L��1��CuSO4��Һ����ת��0.1 mol����ʱ����Һ��pH��________(��������Һ����仯)��
V3����VO2����H2O������ʱ������ӦʽΪ__________________________���ô˵�ص��1 L 1 mol��L��1��CuSO4��Һ����ת��0.1 mol����ʱ����Һ��pH��________(��������Һ����仯)��

 pC��g��+qD��S����H��0��ͼij��Ӧ�����и��������ʵ���n��mol����ʱ��t�ı仯����ͼ��
pC��g��+qD��S����H��0��ͼij��Ӧ�����и��������ʵ���n��mol����ʱ��t�ı仯����ͼ��
