��Ŀ����
����Ŀ����1�������г��˼������ʣ����������ա�
�ٽ��ʯ��������ϩ�� C 60 ���� D �� T �����������������CH4 �� CH3CH2 CH3 ����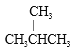 ��CH3CH2CH2CH2CH3����
��CH3CH2CH2CH2CH3����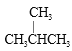 �ͺ� CH3CH2CH2CH3
�ͺ� CH3CH2CH2CH3
����ͬλ�ص���________________������ͬϵ�����_______________������ͬ�����������____________������ͬ ���칹�����______________��
��2����֪һ�������£����ʯת��Ϊʯī�ͷų���������������Ľ��ʯ��ʯī���е�����__________����ߡ������͡������ʽ��ʯ��ʯī�ȶ���______���ǿ���������������������Ľ��ʯ��ʯī���ȼ�վ����ɶ� ����̼��____________�ų������ࡣ
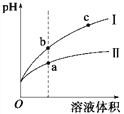
��3��A��B��C�����ձ��зֱ�ʢ����ͬ���ʵ���Ũ�ȵ�ϡ���ᡣ
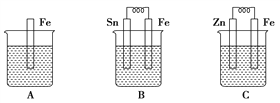
��B �� Sn ���ĵ缫��ӦʽΪ____________________��
Sn ��������Һ�� pH___________________��
��C ���ܷ�Ӧ���ӷ���ʽΪ_________________�� �Ƚ� A��B��C ��������ʴ�����ʣ��ɿ쵽����˳ ����___________��
��4����ͼ�Ǽ���ȼ�ϵ��ԭ��ʾ��ͼ���ش��������⣺
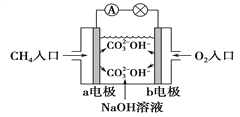
�ٵ�صĸ�����____________(�a����b��)�缫�� �ü��ĵ缫��Ӧ��_______________________��
�� ��ع���һ��ʱ���������Һ�� pH______________��
���𰸡� �� �ܢ� �٢� �� �� �� ���ʯ 2H+��2e-==H2�� ���� Zn��2H+==Zn2+��H2�� B>A>C a CH4��10OH-��8e-==CO![]() ��7H2O ��С
��7H2O ��С
����������1��������ͬ����������ͬ����������ͬ��������ͬһԪ�صIJ�ͬ���ػ�Ϊͬλ�أ�����ͬλ�ص��Ǣڣ��л��������о���ͬһͨʽ����������һ������ij��ԭ���š��ڽṹ�����������ƵĻ�����ϵ�У�����ͬϵ����Ǣܢݣ�ͬ����������ͬ��Ԫ���γɵIJ�ͬ���ʣ����ʯ��"����ϩ"C60������CԪ���γɵIJ�ͬ���ʣ���Ϊͬ�������壻������O2���������O3��������OԪ���γɵIJ�ͬ���ʣ���Ϊͬ�������壬����ͬ����������� �٢ۣ���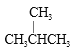 �ͺ� CH3CH2CH2CH3����ʽC4H10��ͬ���ṹ��ͬ����Ϊͬ���칹�壻����ͬ���칹����Ǣޡ���2�����ʯ����ʯī�ͷ�������˵����ͬ���ʵ���ʱ���ʯ�����ߣ����ʯ��˵�������ߣ����ʵ�����Խ��Խ�ȶ�������ʯī�ȶ����������Ľ��ʯ��ʯī���ȼ�վ����ɶ�����̼����ͬ���ʵ����Ľ��ʯ��˵�������ߣ�����ȼ�շ��ȶࡣ��3����B�����������������������������ҺΪ���ᣬ��������ӦΪ2H+��2e-==H2����H�� Ũ�ȼ�С��Sn ��������Һ��pH���ڣ�2��C�γ�ԭ��أ�п�������ã���ԭ��صĸ�������������������ӦΪZn-2e - =Zn2+ ��������ӦΪ2H+��2e-==H2����C ���ܷ�Ӧ���ӷ���ʽΪZn��2H+==Zn2+��H2���� �Ƚ� A��B��C ��������ʴ�����ʣ�A�������ʣ�B��ԭ��صĸ�������죬C����ԭ��ص����������������������ɿ쵽����˳����B>A>C����4���ٻ�ԭ������صĸ�������صĸ�����a�����缫��Ӧ�� CH4��10OH-��8e-==CO
�ͺ� CH3CH2CH2CH3����ʽC4H10��ͬ���ṹ��ͬ����Ϊͬ���칹�壻����ͬ���칹����Ǣޡ���2�����ʯ����ʯī�ͷ�������˵����ͬ���ʵ���ʱ���ʯ�����ߣ����ʯ��˵�������ߣ����ʵ�����Խ��Խ�ȶ�������ʯī�ȶ����������Ľ��ʯ��ʯī���ȼ�վ����ɶ�����̼����ͬ���ʵ����Ľ��ʯ��˵�������ߣ�����ȼ�շ��ȶࡣ��3����B�����������������������������ҺΪ���ᣬ��������ӦΪ2H+��2e-==H2����H�� Ũ�ȼ�С��Sn ��������Һ��pH���ڣ�2��C�γ�ԭ��أ�п�������ã���ԭ��صĸ�������������������ӦΪZn-2e - =Zn2+ ��������ӦΪ2H+��2e-==H2����C ���ܷ�Ӧ���ӷ���ʽΪZn��2H+==Zn2+��H2���� �Ƚ� A��B��C ��������ʴ�����ʣ�A�������ʣ�B��ԭ��صĸ�������죬C����ԭ��ص����������������������ɿ쵽����˳����B>A>C����4���ٻ�ԭ������صĸ�������صĸ�����a�����缫��Ӧ�� CH4��10OH-��8e-==CO![]() ��7H2O��CH4 + 2O2 ��2OH�D �� CO32�D + 3H2O������OH�D ���ʵ�ع���һ��ʱ���������Һ�� pH��С��
��7H2O��CH4 + 2O2 ��2OH�D �� CO32�D + 3H2O������OH�D ���ʵ�ع���һ��ʱ���������Һ�� pH��С��

 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�