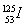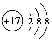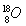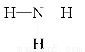��Ŀ����
��1���ڹ̶�������ܱ�������ͨ��N2��H2��������˵���ﵽƽ�����________��
A��3v��N2����v��H2��
B������1��N��N��ͬʱ����6��N��H
C��N2��H2��NH3�����ʵ���֮����1��3��2
D�������������ѹǿ����
E��������ܶȲ���
F�������ƽ����Է�����������
��2����2 L���ܱ�������ͨ��2 mol N2��8 mol H2��5 minʱ�ﵽƽ�⣬���NH3�����ʵ�����2 mol����ƽ��ʱc��H2����______________��
��1��BDF����2��2.5 mol/L
����������1��A��δ˵�����淴Ӧ��B��˵�����淴Ӧ������ȣ�C�в���˵�����ٱ仯������˵���ﵽƽ�⣻D�����ŷ�Ӧ�Ľ��У���������ʵ������٣���ѹǿ��С������ƽ����Է�����������ѹǿ���䣨ƽ����Է����������䣩����˵���ﵽƽ�⣻������������ܶ�ʼ�ղ��䡣��2������3 mol H2����ʣ��5 mol H2����ƽ��ʱ��c��H2����2.5 mol/L��

��ϰ��ϵ�д�
�����Ŀ