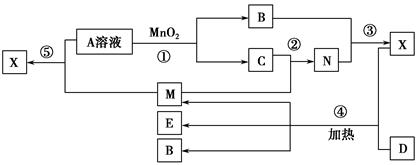
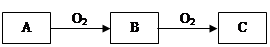��Ŀ����
�ҹ����ٺ����ʡ�ݣ��ܹ�������ú�ˮ�Ƶö��ֻ�����Ʒ����ͼ���Ժ�ˮ�����ǣ���Ҫ�ɷ�CaCO3����Ϊԭ����ȡ���ֻ�����Ʒ�Ĺ�������ͼ������E��һ�ֻ��ʣ�N��һ�ֳ����Ľ������ʡ�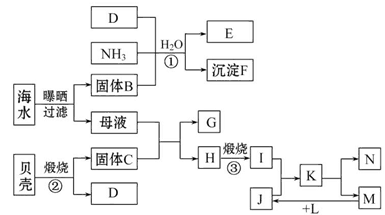
��������������̻ش��������⣺
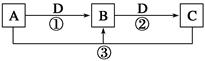
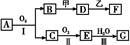
��1������G��L�Ļ�ѧʽ�ֱ�Ϊ___________________��___________________��
��2���������������п���ѭ��ʹ�õ����ʵĻ�ѧʽΪ______________________��
��3����Ӧ�ٵĻ�ѧ����ʽΪ____________���ڷ�Ӧ���б�����ͨ��NH3������ͨ��D����ԭ����_______________________________________________________��
��4����ҵ������F���Ƶ���һ�ֻ�����Ʒ���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��5����K��Һ����δ��������Ƶ�N��_________________________________________________________��
��1��CaCl2 H2 ��2��CO2
��3��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
NH3����Һ�е��ܽ�ȴ���������CO2��ʹ��ת��ΪNH4HCO3
��4��2NaHCO3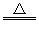 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O
��5��MgCl2��Һ�������Ȼ��������н��������ᾧ�Ƶ�MgCl2���壬���ڵ�����ȡ����þ
����