��Ŀ����
����ѧ���������������ز��������;��ʯ�Ͳ�Ʒ��ϩΪ��ʼԭ�Ͻ��кϳɸ߷��ӻ�����F��G���ϳ�·����ͼ��ʾ��

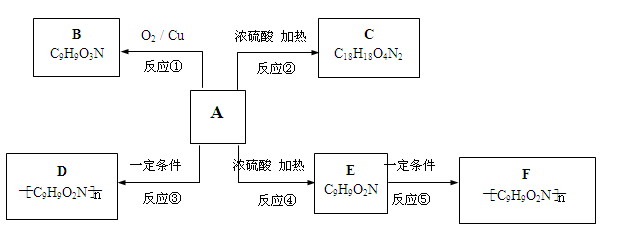
��֪��E�ķ���ʽΪC4H6O2��F�ķ���ʽΪ(C4H6O2)n���������齺������Ҫ�ɷ֣���G�ķ���ʽΪ(C2H4O)n���������ƻ�ѧ��������2CH2=CH2+2CH3COOH+O2 2C4H6O2��������ϩ����+2H2O
2C4H6O2��������ϩ����+2H2O
��֪���� �ṹ���Ƶ��л��ﲻ�ȶ�������������������
�ṹ���Ƶ��л��ﲻ�ȶ�������������������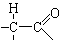
��ش��������⣺
��1��д���ṹ��ʽ��D_______________��F___________________��
��2����Ӧ�١��ڵķ�Ӧ����______________��___________________________��
��3��д�������йط�Ӧ�ĵĻ�ѧ����ʽ��
A����B________________________________________________
B����C _______________________________________________

��֪��E�ķ���ʽΪC4H6O2��F�ķ���ʽΪ(C4H6O2)n���������齺������Ҫ�ɷ֣���G�ķ���ʽΪ(C2H4O)n���������ƻ�ѧ��������2CH2=CH2+2CH3COOH+O2
 2C4H6O2��������ϩ����+2H2O
2C4H6O2��������ϩ����+2H2O��֪����
 �ṹ���Ƶ��л��ﲻ�ȶ�������������������
�ṹ���Ƶ��л��ﲻ�ȶ�������������������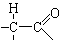
��ش��������⣺
��1��д���ṹ��ʽ��D_______________��F___________________��
��2����Ӧ�١��ڵķ�Ӧ����______________��___________________________��
��3��д�������йط�Ӧ�ĵĻ�ѧ����ʽ��
A����B________________________________________________
B����C _______________________________________________
��1��DΪCH3COOH��FΪ ����2���ӳɷ�Ӧ��ˮ�ⷴӦ��
����2���ӳɷ�Ӧ��ˮ�ⷴӦ��
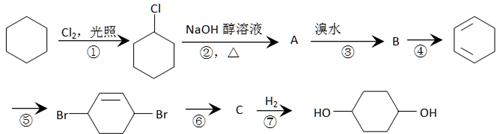
��3��ClCH2CH2Cl+H2O ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH
ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH CH3CHO+NaCl+H2O��
CH3CHO+NaCl+H2O��
 ����2���ӳɷ�Ӧ��ˮ�ⷴӦ��
����2���ӳɷ�Ӧ��ˮ�ⷴӦ����3��ClCH2CH2Cl+H2O
 ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH
ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH CH3CHO+NaCl+H2O��
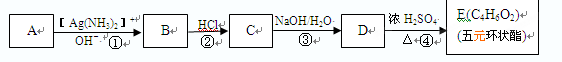
CH3CHO+NaCl+H2O�������������ͼ�����ƶϳ�AΪClCH2CH2Cl��BΪClCH2CH2OH��CΪCH3CHO��DΪCH3COOH��FΪ
 ���ԣ�1���У�DΪCH3COOH��FΪ
���ԣ�1���У�DΪCH3COOH��FΪ ����2���У��١��ڵķ�Ӧ�ֱ�ΪCH2=CH2+Cl2
����2���У��١��ڵķ�Ӧ�ֱ�ΪCH2=CH2+Cl2 ClCH2CH2Cl��
ClCH2CH2Cl��
 (C2H4O)n+nCH3COOH�����䷴Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��ˮ�ⷴӦ����3���У�A����B�ķ�Ӧ����ʽΪClCH2CH2Cl+H2O
(C2H4O)n+nCH3COOH�����䷴Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��ˮ�ⷴӦ����3���У�A����B�ķ�Ӧ����ʽΪClCH2CH2Cl+H2O  ClCH2CH2OH+HCl��B����C�ķ�Ӧ����ʽΪClCH2CH2OH+ NaOH
ClCH2CH2OH+HCl��B����C�ķ�Ӧ����ʽΪClCH2CH2OH+ NaOH CH3CHO+NaCl+H2O��
CH3CHO+NaCl+H2O��������������һ���л��ƶ��⣬�Ǹ߿�������ص㡣���и�������Ϣ���ϴ��������˵���DZȽ����ġ�

��ϰ��ϵ�д�
�����Ŀ
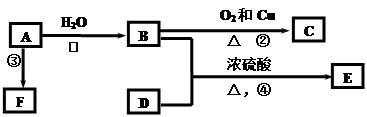
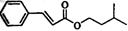 ����һ�����ϣ�һ�ֺϳ�·�����£�
����һ�����ϣ�һ�ֺϳ�·�����£�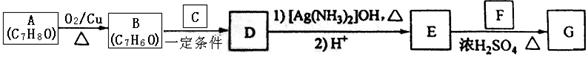
 ��
�� (1)X�ķ���ʽΪ ��
(1)X�ķ���ʽΪ ��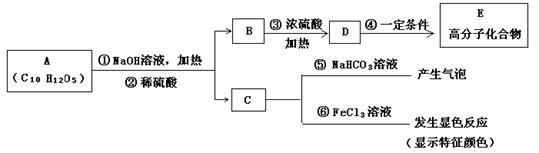

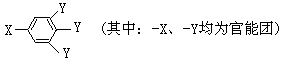
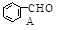 ��CH3CHO
��CH3CHO
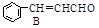 ��H2O
��H2O