��Ŀ����
�̶�������CO2����Ч��������Դ�������ٿ����е��������塣��ҵ����һ����CO2�������״�ȼ�ϵķ�����CO2(g)��3H2(g) CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1
CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1
ij��ѧʵ�齫6molCO2��8molH2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
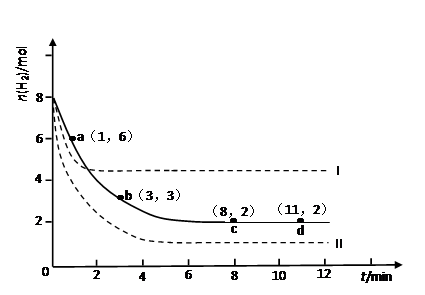
��1��a������Ӧ����_______������ڡ����ڻ�С�ڣ��淴Ӧ���ʡ�
��2������ʱ���ƽ����Ӧ����������___________��
��3�����ı�ijһʵ�������ٽ�������ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı���___________������II��Ӧ��ʵ�������ı���___________��
 CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1
CH3OH(g)��H2O(g) ��H����49.0 kJ��mol��1ij��ѧʵ�齫6molCO2��8molH2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���

��1��a������Ӧ����_______������ڡ����ڻ�С�ڣ��淴Ӧ���ʡ�
��2������ʱ���ƽ����Ӧ����������___________��
| A��0��1min | B��1��3min | C��3��8min | D��8��11min |
��8�֣���1�����ڣ�2�֣���2��A��2�֣� ��3�������¶ȣ�2�֣� ����ѹǿ��2�֣�
�����������1��a��ʱ��û�дﵽƽ��״̬����Ӧ�����������ʵ���������С��ƽ���������ƶ�����������Ӧ���ʴ����淴Ӧ���ʡ�
��2���ֱ���㲻ͬʱ���ڵķ�Ӧ���ʣ�0��1min�ڣ�v��H2����
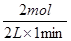 ��1mol/(L?min)����1��3min�ڣ�v��H2����
��1mol/(L?min)����1��3min�ڣ�v��H2����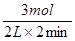 ��0.75mol/(L?min)����3��8min�ڣ�v��H2����
��0.75mol/(L?min)����3��8min�ڣ�v��H2����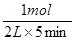 ��0.1mol/(L?min)������ͼ���֪����Ӧ���е�8minʱ���ʵ�Ũ�Ȳ��ٷ����仯����Ӧ�ﵽƽ��״̬������8��11min�ڷ�Ӧ������Ȼ��0.1mol/(L?min)������0��1min�ڷ�Ӧ������ʴ�ΪA��
��0.1mol/(L?min)������ͼ���֪����Ӧ���е�8minʱ���ʵ�Ũ�Ȳ��ٷ����仯����Ӧ�ﵽƽ��״̬������8��11min�ڷ�Ӧ������Ȼ��0.1mol/(L?min)������0��1min�ڷ�Ӧ������ʴ�ΪA����3������I��Ӧ��������ת�������������ʵ����٣�Ӧ���������¶ȣ���÷�Ӧ���ȣ������¶�ƽ�������ƶ���������������ת����������I�������¶ȣ����ߢ�Ӧ��������ת�������������ʵ����࣬������ѹǿƽ�������ƶ�����Ӧ������ѹǿ��
�������������е��Ѷ�����Ŀ��飬Ҳ�Ǹ߿��еij������͡���Ҫ�ǿ���ѧ���Է�Ӧ���ʼ����Լ��������Ӱ�췴Ӧ���ʺ�ƽ��״̬����Ϥ�˽�̶ȣ�ּ������ѧ��������û���֪ʶ���ʵ�����������������������ѧ������˼ά���������Ӧ������������ʱע����ն���Ŀͼ��ķ��������⡣

��ϰ��ϵ�д�
�����Ŀ
 4NO+6H2O(g)����5L���ܱ������н��а���Ӻ�NO�����ʵ���������0.3mol����˷�Ӧ��ƽ�����ʦԣ�x������ʾ��Ӧ����������ʻ���������������ʣ�Ϊ ( )
4NO+6H2O(g)����5L���ܱ������н��а���Ӻ�NO�����ʵ���������0.3mol����˷�Ӧ��ƽ�����ʦԣ�x������ʾ��Ӧ����������ʻ���������������ʣ�Ϊ ( )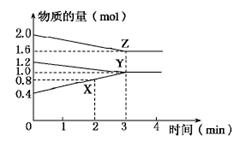
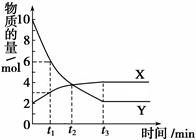

 xC(g)+2D(g)��2minĩ��Ӧ�ﵽƽ��״̬��������0.8mol D�������C��Ũ��Ϊ0.4 mol��L��1������
xC(g)+2D(g)��2minĩ��Ӧ�ﵽƽ��״̬��������0.8mol D�������C��Ũ��Ϊ0.4 mol��L��1������ 2SO3��g�� ��H<0 ��Ӧ��˵��
2SO3��g�� ��H<0 ��Ӧ��˵�� 2NH3(g )��10L���ܱ������н��У����2min�ڣ�N2�����ʵ�����20mol��С��8mol����2min��NH3�ķ�Ӧ����Ϊ(����)
2NH3(g )��10L���ܱ������н��У����2min�ڣ�N2�����ʵ�����20mol��С��8mol����2min��NH3�ķ�Ӧ����Ϊ(����)