��Ŀ����
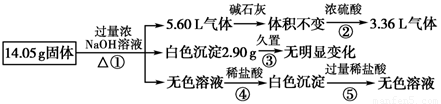
ij�������������Al��(NH4)2SO4��MgCl2��AlCl3��FeCl2�е�һ�ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ(������������ѻ���ɱ�״���µ����)��

�ش��������⣺
(1)��������Ƿ����FeCl2��____________(��ǡ���)��
(2)��������Ƿ����(NH4)2SO4��____________(��ǡ���)��
(3)д����Ӧ�ܵ����ӷ���ʽ��________________________��
(4)����ݼ����жϻ�������Ƿ���AlCl3(�����������ݺͼ���������Ҫ��д�������ļ������)��
������������������ͨ����ʯ���������(����������)����ͨ��Ũ���������С��˵��ʣ��� 6.72 L����Ϊ��������ԭ������һ�����н���Al����������Ϊ ��27 g/mol��5.4 g�����ɰ��������ʵ���Ϊ
��27 g/mol��5.4 g�����ɰ��������ʵ���Ϊ![]() ��0.2 mol����ԭ������һ������ 0.1 mol (NH4)2SO4��������Ϊ13.2 g���õ���ɫ�������ò���ɫ��˵����FeCl2(�����������ױ�����Ϊ����ɫ����������)����ΪNaOH����������ɫ���������ܺ���������������˵��5.8 g��ɫ����ΪMg(OH)2�����MgCl2������Ϊ9.5 g ����ɫ��Һ����Al�����NaOH��Һ��Ӧ��õ���NaAlO2��
��0.2 mol����ԭ������һ������ 0.1 mol (NH4)2SO4��������Ϊ13.2 g���õ���ɫ�������ò���ɫ��˵����FeCl2(�����������ױ�����Ϊ����ɫ����������)����ΪNaOH����������ɫ���������ܺ���������������˵��5.8 g��ɫ����ΪMg(OH)2�����MgCl2������Ϊ9.5 g ����ɫ��Һ����Al�����NaOH��Һ��Ӧ��õ���NaAlO2��
�𰸣�(1)��
(2)��
(3)AlO![]() ��H����H2O===Al(OH)3��
��H����H2O===Al(OH)3��
(4)��������Ϣ���Ƶ�һ������Al��(NH4)2SO4��MgCl2�������ʣ�����������������ʵ�����֮�պõ��� 28.1 g������һ��û��AlCl3��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
����˵����ȷ���ǣ�������
| A������������һ������Al������������ȷ�� | B�����������п��ܺ���MgCl2��AlCl3 | C������������һ������MgCl2��FeCl2 | D������������һ�����У�NH4��2SO4��MgCl2 |