��Ŀ����
��18 gͭ�����Ļ����Ͷ��200 mLϡ������,��ַ�Ӧ��õ���״����2.24 L NO,ʣ��9.6 g����;��������200 mL��Ũ�ȵ�ϡ����,������ȫ�ܽ�,�ֵõ���״����2.24 L NO������Ӧ�����Һ�м���KSCN��Һ,��Һ�����,������˵������ȷ����(����)
| A��ԭ�������ͭ������0.15 mol |
| B��ϡ��������ʵ���Ũ��Ϊ1 mol��L-1 |
| C����һ��ʣ��9.6 g����Ϊͭ���� |
| D���ټ�������200 mLϡ����,����õ���״����2.24 L NO |
A
��һ�μ�����ʱ������ʣ��,��Һ��һ������Fe3+,����1 mol�����μӷ�Ӧ��ʧȥ2 mol����,����2.24 L NOת��0.3 mol����;���Ľ���0.15 mol,����Ϊ18 g-9.6 g="8.4" g,���һ���ܽ�Ľ�����Ħ������Ϊ56 g��mol-1,������ͬ��:�ڶ����ܽ�Ľ�����Ħ������Ϊ64 g��mol-1,��ͭ������3Cu+8HNO3 3Cu(NO3)2+2NO��+4H2O,��֪c(HNO3)=
3Cu(NO3)2+2NO��+4H2O,��֪c(HNO3)= ��4��0.2 L="2" mol��L-1;Dѡ��,�ټ�������,HNO3��0.15 mol Fe2+��Ӧ,���ݵ���غ��֪����0.05 mol NO,����
��4��0.2 L="2" mol��L-1;Dѡ��,�ټ�������,HNO3��0.15 mol Fe2+��Ӧ,���ݵ���غ��֪����0.05 mol NO,����
 3Cu(NO3)2+2NO��+4H2O,��֪c(HNO3)=
3Cu(NO3)2+2NO��+4H2O,��֪c(HNO3)= ��4��0.2 L="2" mol��L-1;Dѡ��,�ټ�������,HNO3��0.15 mol Fe2+��Ӧ,���ݵ���غ��֪����0.05 mol NO,����
��4��0.2 L="2" mol��L-1;Dѡ��,�ټ�������,HNO3��0.15 mol Fe2+��Ӧ,���ݵ���غ��֪����0.05 mol NO,����
��ϰ��ϵ�д�
�����Ŀ

 6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol��
6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol�� ��O2�������жԴ˷�Ӧ��˵���У�����ȷ����(����)
��O2�������жԴ˷�Ӧ��˵���У�����ȷ����(����)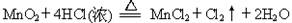
 MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O