��Ŀ����
|
�����ӵ�����ԼΪ6.02��1023 mol��1������˵������ȷ���� | |
A�� |
���³�ѹ�£�16 g 14��CH4����������Ϊ8��6.02��1023 |
B�� |
��⾫��ͭ��ת��0.2��6.02��1023������ʱ�����ܽ�6.4 gͭ |
C�� |
30 g�������辧���к��������ۼ�����ĿΪ2��6.02��1023 |
D�� |
6.2 g����P4(��ͼ)��������P��P���ĸ���Ϊ0.2��6.02��1023
|
�𰸣�C

��ϰ��ϵ�д�
�����Ŀ
�����ӵ�����ԼΪ6.02��1023mol-1������������ȷ���ǣ�������
| A��4 g��ˮ��D2O��������������Ϊ0.2��6.02��1023 | B��2.8 g��ϩ�ͱ�ϩ�Ļ������������̼ԭ����Ϊ0.2��6.02��1023 | C��4.48 L H2��O2�Ļ������������������Ϊ0.2��6.02��1023 | D��0.2 mol Cl2�ܽ��ڵ������ˮ�У�ת�Ƶ�����Ϊ0.2��6.02��1023 |
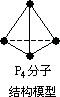 ��
�� �����ӵ�����ԼΪ6.02��1023mol-1������˵����һ����ȷ���ǣ�������
�����ӵ�����ԼΪ6.02��1023mol-1������˵����һ����ȷ���ǣ�������