��Ŀ����
����Ĺ�ҵ�Ʊ���һ����Ҫ�Ļ����������̣���ͬʱ�����������л��������SO2����Ⱦ���1����SO2ͨ��Fe��NO3��3��Һ�У���Һ���ػ�ɫ��Ϊdz��ɫ���������ֱ�Ϊ�ػ�ɫ����ʱ������BaCl2��Һ����������ɫ��������Һ���ػ�ɫ��Ϊdz��ɫ�����ӷ���ʽ��ʾΪ
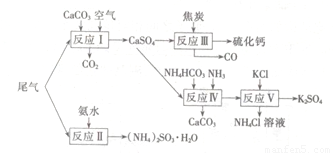
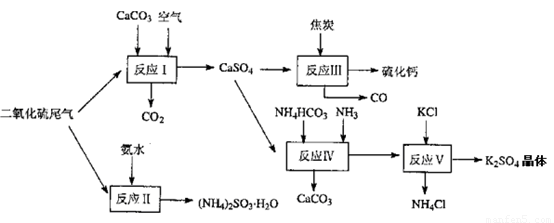
��2�������Ṥҵ��β������ˮ��ʯ��ʯ����̿��̼����狀�KCIΪԭ�Ͽ��Ժϳ�����Ҫ��;���ơ�����ء���������淋����ʣ��ϳ�·����ͼ��
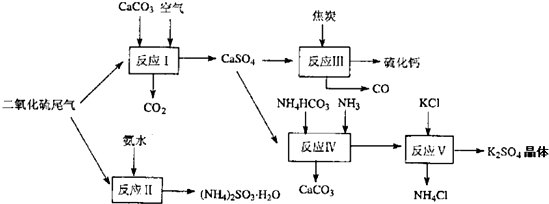
д����Ӧ��Ӧ���Ļ�ѧ����ʽ
��ӦIII���������뻹ԭ�������ʵ���֮��Ϊ
��ӦV��25'C��40%�Ҷ�����Һ�н��У��ø��ֽⷴӦ��˳�����е�ԭ����
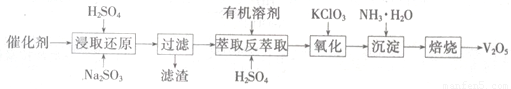
��3���������ײ�����ȱλ����п��ZnFe2Ox������������ʹSO2�ֽ⣬��С��ҵ�����Ի�����Ӱ�죬��������п��ZnFe2O4�������»�ԭ�Ƶã�ת��������ͼ��ʾ��
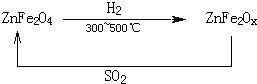
��2molZnFe2Ox��SO2��Ӧ������ 0.75molS��x=
��4��ʯ��ʯ-ʯ��ʪ�����������ռ�����ԭ���������еĶ��������뽬Һ�е�̼����Լ�������Ӧ����ʯ�ࣨCaSO4.2H2O����д���÷�Ӧ�Ļ�ѧ����ʽ
��������1����SO2ͨ��Fe��NO3��3��Һ�У���Һ���ػ�ɫ��Ϊdz��ɫ������������ԭ��Ӧ�����������Ӻ���������ӣ������ֱ�Ϊ�ػ�ɫ������������������������ӵ�������ԭ��Ӧ�����������ӣ�
��2����Ӧ��ΪNH4HCO3��CaSO4��NH3��Ӧ����̼��ƺ�����泥���Ӧ��ѧ����ʽΪ��CaSO4+4C=CaS+4CO����������ΪCaSO4����ԭ��ΪC��������ڲ�ͬ�ܼ����ܽ�ȵIJ�ͬ���Դ˷����ﵽ���������Ŀ�ģ�
��3�����ݵ�ʧ�����غ�ͻ�ѧʽ��Ԫ�ػ��ϼ۴�����Ϊ����㣬����п�����±�������ԭ������ȱλ����п��ˮ��
��4������������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O�����ݹ�ϵʽS��SO2��CaSO4?2H2O�����㣮
��2����Ӧ��ΪNH4HCO3��CaSO4��NH3��Ӧ����̼��ƺ�����泥���Ӧ��ѧ����ʽΪ��CaSO4+4C=CaS+4CO����������ΪCaSO4����ԭ��ΪC��������ڲ�ͬ�ܼ����ܽ�ȵIJ�ͬ���Դ˷����ﵽ���������Ŀ�ģ�
��3�����ݵ�ʧ�����غ�ͻ�ѧʽ��Ԫ�ػ��ϼ۴�����Ϊ����㣬����п�����±�������ԭ������ȱλ����п��ˮ��
��4������������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O�����ݹ�ϵʽS��SO2��CaSO4?2H2O�����㣮
����⣺��1����SO2ͨ��Fe��NO3��3��Һ�У���Һ���ػ�ɫ��Ϊdz��ɫ������������ԭ��Ӧ�����������Ӻ���������ӣ��������ӷ�ӦΪSO2+2Fe3++2H2O=SO42-+2Fe2++4H+�������ֱ�Ϊ�ػ�ɫ������������������������ӵ�������ԭ��Ӧ�����������ӣ����ӷ�ӦΪ3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
�ʴ�Ϊ��SO2+2Fe3++2H2O=SO42-+2Fe2++4H+��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
��2����Ӧ��ΪNH4HCO3��CaSO4��NH3��Ӧ����̼��ƺ�����泥��÷�ӦΪNH4HCO3+CaSO4+NH3=CaCO3��+��NH4��2SO4����Ӧ��ѧ����ʽΪCaSO4+4C=CaS+4CO����������ΪCaSO4����ԭ��ΪC���������뻹ԭ�����ʵ���֮��Ϊ1��4����ӦV��ѡ����40%���Ҷ�����Һ���¶ȿ�����25�棬��ʱ����صIJ��ʳ���90%��ѡ��40%���Ҷ�����Һԭ���������Ҷ�������������ܽ�ȣ�������������
�ʴ�Ϊ��NH4HCO3+CaSO4+NH3=CaCO3��+��NH4��2SO4��1��4��K2SO4���Ҷ�����Һ�е��ܽ��С��
��3���������ײ���ZnFe2OX���ɻ�����ZnFe2O4�����»�ԭ�Ƶã���2mol ZnFe2OX��SO2��Ӧ������0.75molS����ZnFe2OX����ԭΪZnFe2O4��������Ԫ�صĻ��ϼ�Ϊa�����ݵ���ת���غ㣬��֪2mol��2����3-a��=0.75mol��4����ã�a=2.25�����û��ϼ۴�����Ϊ�㣬2+2.25��2=2x�����x=3.25��
����п�����±�������ԭ������ȱλ����п��ˮ���÷�ӦΪ4ZnFe2O4+3H2
4ZnFe2O3.25+3H2O��
�ʴ�Ϊ��3.25��4ZnFe2O4+3H2
4ZnFe2O3.25+3H2O��
��4������������̼��Ʒ�Ӧ������������������̼����Ӧ����ʽΪ��SO2+CaCO3=CaSO3+CO2�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ����ʽΪ��2CaSO3+O2+4H2O=2��CaSO4?2H2O�����ܷ�ӦΪ��2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
S��SO2 ��CaSO4?2H2O
32 172
300t��2.5%��96% m
=
�����m=38.7t��
�ʴ�Ϊ��2CaSO4+2SO2+O2+4H2O=2��CaSO4.2H2O��+2CO2��38.7��
�ʴ�Ϊ��SO2+2Fe3++2H2O=SO42-+2Fe2++4H+��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
��2����Ӧ��ΪNH4HCO3��CaSO4��NH3��Ӧ����̼��ƺ�����泥��÷�ӦΪNH4HCO3+CaSO4+NH3=CaCO3��+��NH4��2SO4����Ӧ��ѧ����ʽΪCaSO4+4C=CaS+4CO����������ΪCaSO4����ԭ��ΪC���������뻹ԭ�����ʵ���֮��Ϊ1��4����ӦV��ѡ����40%���Ҷ�����Һ���¶ȿ�����25�棬��ʱ����صIJ��ʳ���90%��ѡ��40%���Ҷ�����Һԭ���������Ҷ�������������ܽ�ȣ�������������
�ʴ�Ϊ��NH4HCO3+CaSO4+NH3=CaCO3��+��NH4��2SO4��1��4��K2SO4���Ҷ�����Һ�е��ܽ��С��
��3���������ײ���ZnFe2OX���ɻ�����ZnFe2O4�����»�ԭ�Ƶã���2mol ZnFe2OX��SO2��Ӧ������0.75molS����ZnFe2OX����ԭΪZnFe2O4��������Ԫ�صĻ��ϼ�Ϊa�����ݵ���ת���غ㣬��֪2mol��2����3-a��=0.75mol��4����ã�a=2.25�����û��ϼ۴�����Ϊ�㣬2+2.25��2=2x�����x=3.25��
����п�����±�������ԭ������ȱλ����п��ˮ���÷�ӦΪ4ZnFe2O4+3H2
| ||
�ʴ�Ϊ��3.25��4ZnFe2O4+3H2
| ||
��4������������̼��Ʒ�Ӧ������������������̼����Ӧ����ʽΪ��SO2+CaCO3=CaSO3+CO2�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ����ʽΪ��2CaSO3+O2+4H2O=2��CaSO4?2H2O�����ܷ�ӦΪ��2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
S��SO2 ��CaSO4?2H2O
32 172
300t��2.5%��96% m
| 32 |
| 172 |
| 300t��2.5%��96% |
| m |
�ʴ�Ϊ��2CaSO4+2SO2+O2+4H2O=2��CaSO4.2H2O��+2CO2��38.7��
����������Ƚ��ۺϣ��漰������ԭ�����ӷ�Ӧ����ʽ��д����ѧ��Ӧ�ļ���ȣ�����ϰ���е���Ϣ�������Ļ�ѧ��ӦΪ���Ĺؼ���ע��ͼ�����ݵķ��������ã��ϺõĿ���ѧ���������⡢����������������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ��2012?��ͷ��ģ���״���Դ�ḻ���۸�������������淽�㣬��һ����Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ����
��2012?��ͷ��ģ���״���Դ�ḻ���۸�������������淽�㣬��һ����Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ���� ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�