��Ŀ����
���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮��1����֪��2SO2��g��+O2��g��?2SO3��g����H=-Q1kJ?mol-1
2NO��g��+O2��g��?2NO2��g����H=-Q2kJ?mol-1
��ӦNO2��g��+SO2��g��?SO3��g��+NO��g�� �ġ�H=______kJ?mol-1��
��2��һ�������£���NO2��SO2�������1��2�����ܱ������з���������Ӧ�������������Ӧƽ��ʱNO2��NO�����Ϊ1��3����ƽ�ⳣ��K=______��
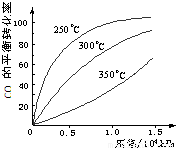
��3��CO�����ںϳɼ״�����Ӧ����ʽΪ��CO��g��+2H2��g��?CH3OH��g����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ���÷�Ӧ��H______0���������������
����֪������һ�ֶ�Ԫ���ᣬ�������ƣ�NaHC2O4����Һ�����ԣ�
��1�������ӷ���ʽ����Na2C2O4��Һ�Լ��Ե�ԭ��______��
��2�������£��Ƚ�0.1mol?L-1NaHC2O4��Һ�и�������Ũ�ȵĴ�С��ϵ______��
��ij����С��Ϊ��̽����BaSO4�ܽ�ȣ��ֱ�����BaSO4���룺a.5ml ˮ��b.40ml 0.2mol?L-1��Ba��OH��2��Һ��c.20ml 0.5mol?L-1��Na2SO4��Һ��d.40ml 0.1mol?L-1��H2SO4��Һ�У��ܽ������ͣ�
��1�����ϸ���Һ�У���Ũ���ɴ�С��˳��Ϊ______��
A��b��a��c��d B��b��a��d��c C��a��d��c��b D��a��b��d��c
��2��ijͬѧȡͬ������Һb����Һdֱ�ӻ�ϣ�������Һ��pHֵΪ______��������Һ�����Ϊ���ǰ����Һ�����֮�ͣ���
���𰸡���������1��2SO2��g��+O2��g��?2SO3��g����H=-Q1kJ?mol-1 ��
2NO��g��+O2��g��?2NO2��g����H=-Q2kJ?mol-1 ��
������ʽ ���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g�����ʱ������Ӧ�ĸı䣻
���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g�����ʱ������Ӧ�ĸı䣻
��2������ƽ����ϵ�и�������������������ƽ�ⳣ����
��3�������¶�ƽ�������ȷ�Ӧ�����ƶ������һ����̼��ת���ʺ��¶ȵĹ�ϵͼƬ�����жϣ��Ӷ�ȷ����Ӧ���ʱ䣻
��1��Na2C2O4��ǿ�������Σ�ˮ�����Һ�ʼ��ԣ�
��2��������Һ��������ӵĵ����ˮ��ȷ����Һ�и������ӵ�Ũ�ȹ�ϵ��
��1��������ͬ���������������ᱵ���ܽ⣬������������������ʽ���ת����
��2���ȼ�������Һ������������Ũ�ȣ��ٸ������ӻ���ʽ����������Ũ�ȣ��Ӷ�ȷ����Һ��pHֵ��
����⣺��1��2SO2��g��+O2��g��?2SO3��g����H=-Q1kJ?mol-1 ��
2NO��g��+O2��g��?2NO2��g����H=-Q2kJ?mol-1 ��
������ʽ ���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g����H=
���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g����H= ��
��
�ʴ�Ϊ�� ��
��
��2��NO2��g��+SO2��g��?SO3��g��+NO��g�����跴Ӧ��ʼʱ�������������Ϊx��������������Ϊ2x����Ӧ�ﵽƽ��״̬ʱ�������������Ӧ�����Ϊy���÷�Ӧ�ж���������һ��������Ӧ�������Ϊ1��1���������ɵ�һ�����������Ϊy��ƽ��ʱNO2��NO�����Ϊ1��3������y= x����ƽ��ʱ���������������=x-
x����ƽ��ʱ���������������=x- =
= x��������������=2x-
x��������������=2x- ��һ�����������=
��һ�����������= x��������������=
x��������������= x����ƽ�ⳣ��=
x����ƽ�ⳣ��= =1.8���ʴ�Ϊ��1.8��
=1.8���ʴ�Ϊ��1.8��
��3�������¶ȣ�һ����̼��ת���ʽ��ͣ�ƽ�����淴Ӧ�����ƶ������淴Ӧ���������ȷ�Ӧ�����H��0���ʴ�Ϊ������
��1�����������ᣬ��������ǿ����������ˮ�����Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ��Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��C2O42-+H2O?HC2O4-+OH-��
�ʴ�Ϊ��C2O42-+H2O?HC2O4-+OH-��
��2��NaHC2O4��Һ�������Ӳ�ˮ�⣬HC2O4-ˮ���������Ũ�ȱȴ�HC2O4-��HC2O4-�����ˮ�⣬NaHC2O4��Һ������˵��HC2O4-����̶ȴ���ˮ��̶ȣ���Һ��������Ũ�ȴ�������������Ũ�ȣ�HC2O4-����������Ӻ�ˮ����������ӵ���������Ũ�ȴ���C2O42-Ũ�ȣ����Ե���Ϊ��ˮ��Ϊ�Σ�����c��HC2O4-����c��H+����������Һ�и�������Ũ�ȵĴ�С��ϵΪ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-�����ʴ�Ϊ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
��1���������ܵ���ʵ��ܶȻ�����֪����Һ�����������Ũ��Խ�����ᱵ���ܽ��ԽС��������Ũ��Խ�ͣ������������������ᱵ�ĵ��룬������������Һ�к��б����ӣ����Ա�����Ũ�����ˮ�еı�����Ũ�ȴ�֮����������Һ��������Һ�ж�������������ӣ��������ᱵ�ĵ��룬�������е������Ũ�ȴ��������е�Ũ�ȣ�������������Һ�б����ӵ�Ũ��С��������Һ�б�����Ũ�ȣ����Ա�����Ũ�ȴ�С˳���ǣ�b��a��d��c����ѡB��
��2��40ml 0.2mol?L-1��Ba��OH��2��Һ��40ml 0.1mol?L-1��H2SO4��Һ�л�Ϻ���Һ��C��OH-��= =0.1mol/L����C��H+��=10-13 mol/L������pH=13���ʴ�Ϊ��13��
=0.1mol/L����C��H+��=10-13 mol/L������pH=13���ʴ�Ϊ��13��
���������⿼������Һ�и�������Ũ�ȴ�С�ıȽϡ������ҺpHֵ�ļ��㡢ƽ�ⳣ���ļ����֪ʶ�㣬�ѶȽϴ�ע���������Һ��pHֵ�ļ���ʱ��Һ�����Ϊ������������ᵼ�´���
2NO��g��+O2��g��?2NO2��g����H=-Q2kJ?mol-1 ��
������ʽ
 ���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g�����ʱ������Ӧ�ĸı䣻
���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g�����ʱ������Ӧ�ĸı䣻��2������ƽ����ϵ�и�������������������ƽ�ⳣ����
��3�������¶�ƽ�������ȷ�Ӧ�����ƶ������һ����̼��ת���ʺ��¶ȵĹ�ϵͼƬ�����жϣ��Ӷ�ȷ����Ӧ���ʱ䣻
��1��Na2C2O4��ǿ�������Σ�ˮ�����Һ�ʼ��ԣ�
��2��������Һ��������ӵĵ����ˮ��ȷ����Һ�и������ӵ�Ũ�ȹ�ϵ��
��1��������ͬ���������������ᱵ���ܽ⣬������������������ʽ���ת����
��2���ȼ�������Һ������������Ũ�ȣ��ٸ������ӻ���ʽ����������Ũ�ȣ��Ӷ�ȷ����Һ��pHֵ��
����⣺��1��2SO2��g��+O2��g��?2SO3��g����H=-Q1kJ?mol-1 ��
2NO��g��+O2��g��?2NO2��g����H=-Q2kJ?mol-1 ��
������ʽ
 ���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g����H=
���÷���ʽNO2��g��+SO2��g��?SO3��g��+NO��g����H= ��
���ʴ�Ϊ��
 ��
����2��NO2��g��+SO2��g��?SO3��g��+NO��g�����跴Ӧ��ʼʱ�������������Ϊx��������������Ϊ2x����Ӧ�ﵽƽ��״̬ʱ�������������Ӧ�����Ϊy���÷�Ӧ�ж���������һ��������Ӧ�������Ϊ1��1���������ɵ�һ�����������Ϊy��ƽ��ʱNO2��NO�����Ϊ1��3������y=
 x����ƽ��ʱ���������������=x-
x����ƽ��ʱ���������������=x- =
= x��������������=2x-
x��������������=2x- ��һ�����������=
��һ�����������= x��������������=
x��������������= x����ƽ�ⳣ��=
x����ƽ�ⳣ��= =1.8���ʴ�Ϊ��1.8��
=1.8���ʴ�Ϊ��1.8����3�������¶ȣ�һ����̼��ת���ʽ��ͣ�ƽ�����淴Ӧ�����ƶ������淴Ӧ���������ȷ�Ӧ�����H��0���ʴ�Ϊ������
��1�����������ᣬ��������ǿ����������ˮ�����Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ��Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��C2O42-+H2O?HC2O4-+OH-��
�ʴ�Ϊ��C2O42-+H2O?HC2O4-+OH-��
��2��NaHC2O4��Һ�������Ӳ�ˮ�⣬HC2O4-ˮ���������Ũ�ȱȴ�HC2O4-��HC2O4-�����ˮ�⣬NaHC2O4��Һ������˵��HC2O4-����̶ȴ���ˮ��̶ȣ���Һ��������Ũ�ȴ�������������Ũ�ȣ�HC2O4-����������Ӻ�ˮ����������ӵ���������Ũ�ȴ���C2O42-Ũ�ȣ����Ե���Ϊ��ˮ��Ϊ�Σ�����c��HC2O4-����c��H+����������Һ�и�������Ũ�ȵĴ�С��ϵΪ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-�����ʴ�Ϊ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
��1���������ܵ���ʵ��ܶȻ�����֪����Һ�����������Ũ��Խ�����ᱵ���ܽ��ԽС��������Ũ��Խ�ͣ������������������ᱵ�ĵ��룬������������Һ�к��б����ӣ����Ա�����Ũ�����ˮ�еı�����Ũ�ȴ�֮����������Һ��������Һ�ж�������������ӣ��������ᱵ�ĵ��룬�������е������Ũ�ȴ��������е�Ũ�ȣ�������������Һ�б����ӵ�Ũ��С��������Һ�б�����Ũ�ȣ����Ա�����Ũ�ȴ�С˳���ǣ�b��a��d��c����ѡB��
��2��40ml 0.2mol?L-1��Ba��OH��2��Һ��40ml 0.1mol?L-1��H2SO4��Һ�л�Ϻ���Һ��C��OH-��=
 =0.1mol/L����C��H+��=10-13 mol/L������pH=13���ʴ�Ϊ��13��
=0.1mol/L����C��H+��=10-13 mol/L������pH=13���ʴ�Ϊ��13�����������⿼������Һ�и�������Ũ�ȴ�С�ıȽϡ������ҺpHֵ�ļ��㡢ƽ�ⳣ���ļ����֪ʶ�㣬�ѶȽϴ�ע���������Һ��pHֵ�ļ���ʱ��Һ�����Ϊ������������ᵼ�´���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2011?ɽ�����о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壮
��2011?ɽ�����о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壮
 ���£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г������������Իش���������
���£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г������������Իش��������� ���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮
���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮