��Ŀ����
ͼ���ǻ�ѧʵ�����г����Ʊ�����������IJ�������װ�á�ijѧУͬѧ������ѧ�����Լ��������������ʵ�顣
 |

ͼ��
��1��ͼ��������B�����ƣ�_______________________��
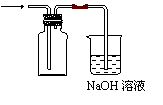
��2��ͬѧ������ͼ��װ���Ʊ����ռ������NO2���壬���ڷ����ڻ����ü���ƿ�ռ�NO2��װ��ͼ��B�з�����Ӧ�����ӷ���ʽΪ____________________________________��
��3��ͬѧ������ͼ��װ��ͨ��������Ӧǰ��C��������ȷ��Na2CO3��NaCl����������Na2CO3��������A�м���ϡ���ᣬB�м���Na2CO3��NaCl�������C�м����ʯ�ҡ���װ�ô��ڽ϶�ȱ�ݣ��Ӷ�����ʵ�����ϴ�����˵�����е�����ȱ�ݣ�
��_________________________________________________________________________��
��_________________________________________________________________________��
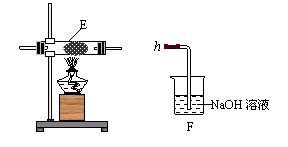
 ��4��ͬѧ������ͼ��װ����ȡ�����������Ļ�����壬��������ͼ��װ����֤����ijЩ���ʡ�A�м���Ũ��ˮ��C�м����ʯ�ң�E�ڷ��ô���(��ʯ��)���������������Ӹ�����a b
c h��
��4��ͬѧ������ͼ��װ����ȡ�����������Ļ�����壬��������ͼ��װ����֤����ijЩ���ʡ�A�м���Ũ��ˮ��C�м����ʯ�ң�E�ڷ��ô���(��ʯ��)���������������Ӹ�����a b
c h��
ͼ��
��B������������Լ�������Ϊ____________________��B���ܲ���������������������ԭ����___________________________________________________________________��
��ʵ���й۲쵽E���к���ɫ������֣�֤����������_________�ԣ�E�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________________________________��
��1��Բ����ƿ ��2�֣�
 ��2��
��2��
��û��β������װ�ò��۷֣� ��2�֣�
Cu + 4H�� + 2NO3������ Cu2�� + 2NO2��+ 2H2O ��2�֣�
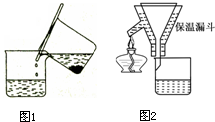
��3��û������Բ����ƿ��ˮ������װ�ã����CO2�л��е�ˮ����Ҳ�ᱻC���գ���������������ͨ�������е�CO2��ˮ����Ҳ�ᱻ���գ���Ӧ������װ���ڲ�����CO2������ȫ�ų����ȵȡ����𰸺��������֣� ������������ ��ÿ��1�֣���2�֣�
��4���ٹ������� ��2�֣�
����������Ũ��ˮ�е�H2O��Ӧ�����������ƺ�������ͬʱ�ų������ȣ��¶�����ʹ�����ܽ�ȼ�С���ݳ����������Ƶ������OH�������˰�ˮ��OH��Ũ�ȣ���ʹ��ˮ����ƽ�����ƣ����°����ų� ��2�֣�
 �ڻ�ԭ��
��2�֣�
�ڻ�ԭ��
��2�֣�
4NH3 + 5O2 ===== 4NO + 6H2O 2NO + O2 ���� 2NO2 ����2�֣���4�֣�

 ���������[��NH4��2SO4?FeSO4?6H2O]Ϊdz��ɫ���壬������ˮ�������ھƾ�����ˮ�е��ܽ�ȱ�FeSO4��NH4��2SO4��ҪС��ʵ�����г��Է���мΪԭ�����Ʊ����䲽�����£�
���������[��NH4��2SO4?FeSO4?6H2O]Ϊdz��ɫ���壬������ˮ�������ھƾ�����ˮ�е��ܽ�ȱ�FeSO4��NH4��2SO4��ҪС��ʵ�����г��Է���мΪԭ�����Ʊ����䲽�����£�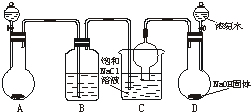 ��°��������ҹ����������Ļ���ר�ң������Ƽ���������ش��ף����������������Ƽ�����������Ƽ������ͼ����ʵ������ģ�⡰�����Ƽ������ȡ̼������һ����ʵ��װ�ã�ʵ�鲽��Ϊ��
��°��������ҹ����������Ļ���ר�ң������Ƽ���������ش��ף����������������Ƽ�����������Ƽ������ͼ����ʵ������ģ�⡰�����Ƽ������ȡ̼������һ����ʵ��װ�ã�ʵ�鲽��Ϊ��


