��Ŀ����
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��________(�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ______��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ________����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ________���õ���ܷ�Ӧ�����ӷ���ʽΪ________��
(1)n(Cl)��0.0250 L��0.40 mol��L��1��0.010 mol,0.54 g��0.010 mol��35.5 g��mol��1��0.185 g��n(Fe)��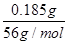 ��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3
��0.0033 mol��n(Fe)��n(Cl)��0.0033��0.010��1��3��x��3
(2)0.1��HCl��Cl2
(3)2Fe3����2I��=2Fe2����I2��(4)2Fe(OH)3��4OH����3ClO��=2FeO42-��3Cl����5H2O��
FeO42-��3e����4H2O=Fe(OH)3��5OH����
3Zn��2FeO42-��8H2O=3Zn(OH)2��2Fe(OH)3��4OH��
����

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д���15�֣�ʵ��̽����ѧϰ��ѧ��һ����Ҫ������ijʵ��С���ͬѧ��������װ�����һЩ���������Ʊ��Լ������������̽�����г�װ�ü���������ʡ�ԣ�����װ��E�ж����ʹ�ã���
�ɹ�ѡ���Һ���Լ�������ҩƷ��
| Һ���Լ� | ����ҩƷ |
| ϡ���ᡢϡ���ᡢϡ���ᡢNaOH��Һ��Ũ��ˮ��5%H2O2��Һ��Ũ���ᡢ����ʳ��ˮ | CaCO3��CaO��MnO2��KMnO4��CaC2�� ��ʯ�ҡ�Cu��Zn��Na2S |
��1��װ��B��a���������� ��
��2������Aװ����ȡ��Է�������С��32�������� ������д2�֣���
��3����֪�����������ڳ����¿��Է���������ԭ��Ӧ��A��B�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백���ķ�Ӧװ�ã�������������˳����A�� ��C�� �� ��B��д��װ��B��������ȣ��з�Ӧ�����ӷ���ʽ ��
��4���������ʵ����ѡ������D��E��D��ѡ�ã� ����E��ѡ�ã� ������д��ţ�
A����ˮ�Ȼ��� B������̼��������Һ�� C������ʳ��ˮ��
D�����������ס� E����ʯ�� F��Ũ����
��5��C�ڳ��ִ������̣���һ�������ﳣ����ʳƷ��װ���ڵı���������д����Ӧ�Ļ�ѧ����ʽ ��
��6������������ѧ��ѧ֪ʶ���һ��ʵ�鷽��������������е�����
�������������������м�����Ҫ�����ã��������й��ա���Ч���Ͷ���ɱ�������������ǽ��չ�����ơ�
(1)Cl2��H2O2��ClO2(��ԭ����ΪCl��)��O3(1 mol O3ת��Ϊ1 mol O2��1 mol H2O)�����ʳ��������������������ʵ�����������������Ч����ߵ���________(�����)��
| A��Cl2 | B��H2O2 | C��ClO2 | D��O3 |
(3)Ư����������(NaClO2)�ڳ�����ڰ����ɱ���һ�ꡣ������ȶ��ɷֽ⣬��Ӧ�����ӷ���ʽΪHClO2�D��ClO2����H����Cl����H2O(δ��ƽ)���ڸ÷�Ӧ�У�����1 mol ClO2����ʱת�Ƶĵ��Ӹ���ԼΪ________��
(4)��84������Һ(��Ҫ�ɷ���NaClO)�ͽ��(��Ҫ�ɷ���Ũ����)���ܻ��ã�ԭ����__________________(�����ӷ���ʽ��ʾ)�������ȼҵ�IJ������������84������Һ��д���йط�Ӧ�Ļ�ѧ����ʽ��____________��
ij�����Һ�п��ܺ��е��������±���ʾ:
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH4+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32����AlO2�� |
Ϊ̽����ɷ�,����������̽��ʵ�顣
(1)̽��һ:
��ͬѧȡһ�����Ļ����Һ,��������μ�������������Һ,�������������ʵ���(n)���������������Һ�����(V)�Ĺ�ϵ��ͼ��ʾ��
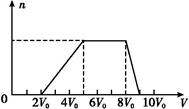
�ٸ���Һ��һ�������ڵ����������� ,
һ�������ڵ�������������������������;���е����������Ӧ���ʵ���Ũ��֮��Ϊ�� ;
����д���������ٹ����з�����Ӧ�����ӷ���ʽ ��
(2)̽����:
��ͬѧ������Һ�к��д�����Cl-��Br-��I-,����1 L�û����Һ��ͨ��һ������Cl2,��Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2�����(��״��)�Ĺ�ϵ���±���ʾ,������ش���������:
| Cl2�����(��״��) | 11.2 L | 22.4 L | 28.0 L |
| n(Cl-) | 2.5 mol | 3.5 mol | 4.0 mol |
| n(Br-) | 3.0 mol | 2.5 mol | 2.0 mol |
| n(I-) | x mol | 0 | 0 |
�ٵ���ʼ��ͨ��Cl2�����Ϊ22.4 Lʱ,��Һ�з�����Ӧ�ܵ����ӷ���ʽΪ�� ;
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ�� ��

 ����֪����Һ����Al2O3��Ӧ����
����֪����Һ����Al2O3��Ӧ����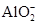 ���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��
���ɣ���ԭ��Һ��һ������_______�����ܺ��д�����_______��