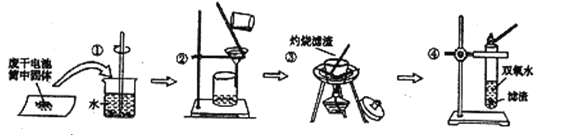
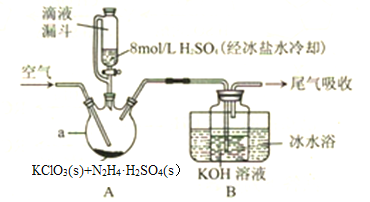
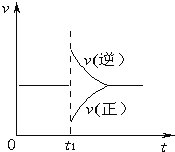
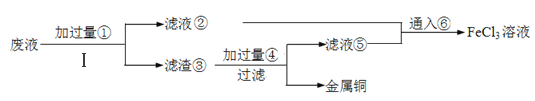
��Ŀ����
����Ŀ��ˮ�ĵ���ƽ��������ͼ��ʾ��
��1����A�㡢B��ֱ��ʾ25���100��ʱˮ�ĵ���ƽ��ʱ���ӵ�Ũ�ȣ����ʾ25�����______������A������B������100��ʱˮ�����ӻ�Ϊ__________��
��2��100�棬��pH=9��NaOH��Һ��pH=4��������Һ��ϣ������û����ҺpH=7����NaOH��Һ��������Һ�������Ϊ_____��

��3��������ԭ�ζ�ʵ��ͬ�к͵ζ����ƣ�����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮��������0.001mol/L����KMnO4��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ���ӷ���ʽ��2MnO4-+ 5HSO3- + H+=2Mn2+ + 5SO42- + 3H2O ��ջش�������

���ζ�ʱ��������KMnO4��Һװ��ͼ�е�___________ (����������������) �ζ����С�
����ʵ��___________ (������Ҫ����������Ҫ��)ָʾ����
�����в�����ʹ�ⶨ���ƫ�ߵ���_____
A.��ƿϴ������������ˮ
B.����KMnO4��Һ�ε���ƿ��
C.ʢװKMnO4��Һ�ĵζ��ܵζ�ǰ������ȷ���ζ������Ӷ���
���𰸡� A 10-12 1:9 �� ����Ҫ BC
��������(1)A��c(H+)=c(OH-)=10-7 mol/L��Kw=c(H+)c(OH-)=10-14 ����A��Ϊ25����B��c(H+)=c(OH-)=10-6 mol/L��Kw=c(H+)c(OH-)=10-12 ����B��Ϊ100����100��ʱ1molL-1��NaOH��Һ��Kw=c(H+)c(OH-)=10-12 ��c(OH-)=1mol/L����������������ˮ�ĵ��룬��Һ�е���������ˮ�ĵ���ģ���ˮ�������c(H+)=1��10-12���ʴ�Ϊ��A��1��10-12��
(2)100��ʱKw=c(H+)c(OH-)=10-12 ��pH=4��������Һ��������Ũ��Ϊ1��10-4molL-1��pH=9��NaOH��Һ������������Ũ��Ϊ�� ![]() mol/L=1��10-3molL-1����Ϻ�pH=7����Һ�ʼ��ԣ���Ӧ����Һ������������Ũ��Ϊ��
mol/L=1��10-3molL-1����Ϻ�pH=7����Һ�ʼ��ԣ���Ӧ����Һ������������Ũ��Ϊ�� ![]() mol/L=1��10-5molL-1����NaOH��Һ��������Һ������ֱ�Ϊx��y����
mol/L=1��10-5molL-1����NaOH��Һ��������Һ������ֱ�Ϊx��y����![]() =1��10-5�����x��y=1��9���ʴ�Ϊ��1��9��
=1��10-5�����x��y=1��9���ʴ�Ϊ��1��9��
(3)�����Ը�����ؾ���ǿ�����ԣ�����ʴ�ܣ�����Ҫװ����ʽ�ζ����ڣ��ʴ�Ϊ���ף�
�� ��ʵ���и��������Һ�������Ե���ɫ���ڷ�Ӧ�����������Ե���ɫ�仯������Ҫʹ��ָʾ�������жϵζ��յ㣬�ʴ�Ϊ������Ҫ��
��A.��ƿϴ������������ˮ����Ӱ�����ʵ����ʵ�������ʵ����Ӱ�죬����B.����KMnO4��Һ�ε���ƿ�⣬�������ĵ�KMnO4��Һ�����ƫ�࣬�ⶨ���ƫ�ߣ���ȷ��C.ʢװKMnO4��Һ�ĵζ��ܵζ�ǰ������ȷ���ζ������Ӷ��������¶���ƫ�����ĵ�KMnO4��Һ�����ƫ�࣬�ⶨ���ƫ�ߣ���ȷ����ѡBC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ͬ���ܱ������У��øߴ�������Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺2 H2O(g)![]() 2 H2(g) + O2(g) H>0��ʵ���÷�Ӧ��ϵ��ˮ����Ũ�ȣ�mol/L���ı仯������£�
2 H2(g) + O2(g) H>0��ʵ���÷�Ӧ��ϵ��ˮ����Ũ�ȣ�mol/L���ı仯������£�
��� | ʱ��/min | 0 | 10 | 20 | 30 | 40 | 60 |
�� | �¶�T1 / 1��Cu2O | 0.0500 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
�� | �¶�T1 / 2��Cu2O | 0.0500 | 0.0490 | 0.0483 | 0.0480 | 0.0480 | 0.0480 |
�� | �¶�T2 / 2��Cu2O | 0.0500 | 0.0480 | 0.0470 | 0.0470 | 0.0470 | 0.0470 |
����˵������ȷ����
A. ʵ���ǰ20 min��ƽ����Ӧ����v(O2) = 7��10��5 mol/( L��min)
B. ʵ������������µ�ƽ�ⳣ����ȣ���С��ʵ��������µ�ƽ�ⳣ��
C. 2��Cu2O�Ĵ�Ч�ʱ�1��Cu2O�Ĵ�Ч�ʸ�
D. ʵ��ʱ���¶�T2����T1