��Ŀ����
�þ���ȷ������NaOH��������250mL 0.2mol?L-1 NaOH��Һ��
��1��������ʵ�������У�����ʹ�õ���______������ţ���
A��250mL�ձ� B��500mL����ƿ�� C���Լ�ƿ�������� D��������ƽ���� E����ͷ�ι�
��2�������������п�ʹ�õ����⣬��ȱ�ٵ���Ҫ������______��
��3��ʹ������ƿǰ������е�һ��������______�����⣬������ƿ�϶�����______��______��______����������ǣ�
��4������ʱ�����²������裬��ȷ��˳����______��
A��������������ˮ����ʢNaOH���ձ�����ȫ�ܽ⣬��ȴ������
B�����ձ��е���ҺС��ת�Ƶ�����ƿ��
C������������ƿ�м�����ˮ��Һ���̶���1cm��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��ײ���̶�������
D������������ˮϴ���ձ��Ͳ�����2�Ρ�3�Σ�ÿ��ϴ��Һ��С��ע������ƿ����������
E��������ƿ�����������ҡ��
��5�����������ʹ������ҺŨ��ƫ�ߵ���______��
a��û�н���������������D
b��������ˮ���������̶���
c��������������
d��δ��ȴ�����¾�ת��������ƿ���ݣ�
�⣺��1�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�ת�Ƶ��Լ�ƿ�У�����ǩ���棬��������������������ƽ���ձ�����������250mL����ƿ����ͷ�ιܡ�ҩ�ף�
�ʲ���Ҫ������Ϊ��B��500mL����ƿ D��������ƽ��
�ʴ�Ϊ��BD��
��2���ɣ�1���ṩ��������֪������������250ml������ƿ����������
�ʴ�Ϊ��250ml������ƿ����������
��3����Һ���������Ҫ�������µߵ�ҡ�ȣ���ʹ������ƿ֮ǰ��Ҫ�������ƿ�Ƿ�©ˮ������ƿ�϶������¶ȡ��������̶��ߣ�
�ʴ�Ϊ���������ƿ�Ƿ�©ˮ���¶ȣ��������̶��ߣ�
��4���ɣ�1����ʵ�����˳���֪����ȷ��˳����ABDCE���ʴ�Ϊ��ABDCE��
a��δϴ���ձ�����������������������մ���ձ����벣�����ϣ��������Ƶ�ʵ��������С����ҺŨ��ƫ�ͣ���a�����ϣ�
b��������ˮ���������̶��ߣ���Һ�����ƫ��������Һ��Ũ��ƫ�ͣ���b�����ϣ�
c�������������ʣ�ʵ�ʳ������������Ƶ�����ƫ��������Һ��Ũ��ƫ�ߣ���c���ϣ�
d��δ��ȴ�����¾�ת��������ƿ���ݣ���Һ��ȴ��������Һ�����ƫС����Һ��Ũ��ƫ�ߣ���d���ϣ�
�ʴ�Ϊ��cd��
��������1������������Һ�IJ�������ѡ������������
��2������������Һ�IJ�������ѡ�����������������ж�ȱ�ٵ�������
��3����Һ���������Ҫ�������µߵ�ҡ�ȣ���ʹ������ƿ֮ǰ��Ҫ�������ƿ�Ƿ�©ˮ������ƿ�϶������¶ȡ��������̶��ߣ�
��4������������Һ�IJ������裬���в�����������
��5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=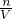 ��������ҺŨ�ȵ�Ӱ�죮
��������ҺŨ�ȵ�Ӱ�죮
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע�����c=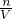 ������Һ������ԭ������������
������Һ������ԭ������������
�ʲ���Ҫ������Ϊ��B��500mL����ƿ D��������ƽ��
�ʴ�Ϊ��BD��
��2���ɣ�1���ṩ��������֪������������250ml������ƿ����������
�ʴ�Ϊ��250ml������ƿ����������
��3����Һ���������Ҫ�������µߵ�ҡ�ȣ���ʹ������ƿ֮ǰ��Ҫ�������ƿ�Ƿ�©ˮ������ƿ�϶������¶ȡ��������̶��ߣ�
�ʴ�Ϊ���������ƿ�Ƿ�©ˮ���¶ȣ��������̶��ߣ�
��4���ɣ�1����ʵ�����˳���֪����ȷ��˳����ABDCE���ʴ�Ϊ��ABDCE��
a��δϴ���ձ�����������������������մ���ձ����벣�����ϣ��������Ƶ�ʵ��������С����ҺŨ��ƫ�ͣ���a�����ϣ�
b��������ˮ���������̶��ߣ���Һ�����ƫ��������Һ��Ũ��ƫ�ͣ���b�����ϣ�
c�������������ʣ�ʵ�ʳ������������Ƶ�����ƫ��������Һ��Ũ��ƫ�ߣ���c���ϣ�
d��δ��ȴ�����¾�ת��������ƿ���ݣ���Һ��ȴ��������Һ�����ƫС����Һ��Ũ��ƫ�ߣ���d���ϣ�
�ʴ�Ϊ��cd��
��������1������������Һ�IJ�������ѡ������������
��2������������Һ�IJ�������ѡ�����������������ж�ȱ�ٵ�������
��3����Һ���������Ҫ�������µߵ�ҡ�ȣ���ʹ������ƿ֮ǰ��Ҫ�������ƿ�Ƿ�©ˮ������ƿ�϶������¶ȡ��������̶��ߣ�
��4������������Һ�IJ������裬���в�����������
��5���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
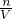 ��������ҺŨ�ȵ�Ӱ�죮
��������ҺŨ�ȵ�Ӱ�죮���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע�����c=
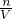 ������Һ������ԭ������������
������Һ������ԭ������������ 
��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ