��Ŀ����
�������е�һ����ԭ�ӱ���ȡ�������üװ���CH3NH2���������백���ƣ�CH3NH2��H2OҲ��һԪ���25��ʱ���볣��Kb=4.0��10-5������0.0500mol/L��ϡ����ζ�l0mL 0.1000mol/L�ļװ���Һ����Һ��c(OH-)�ĸ�������pOH��������ϡ����������V���Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
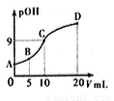
A. �װ���ˮ�еĵ��뷽��ʽΪ��CH3NH2��H2O=CH3NH3++OH-�������Һ�ʼ���
B. C��������Һ�� =2.5��10-5
=2.5��10-5
C. A��B��C��D�ĵ���Һ�У�ˮ���������c(H+):C > D > B > A
D. B����Һ�д���c(CH3NH2��H2O)>c(CH3NH3+)>c(H+)>c(OH-)
��ϰ��ϵ�д�
�����Ŀ


 HFeCl4 ���Իش��������⣺
HFeCl4 ���Իش��������⣺ 2SO2(g)��O2(g)���ﵽƽ�⣻�����¶Ȳ��䣬�ٳ�����ͬ�����SO3(g)���ﵽ��ƽ�����ԭƽ����ȣ�����ֵ��С���� ( )
2SO2(g)��O2(g)���ﵽƽ�⣻�����¶Ȳ��䣬�ٳ�����ͬ�����SO3(g)���ﵽ��ƽ�����ԭƽ����ȣ�����ֵ��С���� ( )


 ������K
������K Ca2��(aq)��2OH��(aq) ��H<0�������йظ�ƽ����ϵ��˵����ȷ���ǣ� ��
Ca2��(aq)��2OH��(aq) ��H<0�������йظ�ƽ����ϵ��˵����ȷ���ǣ� ��