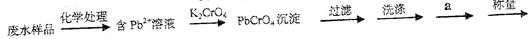��Ŀ����
��ش��й����⣺
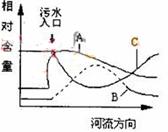
��ͼ�л�����������߷ֱ��������������������ˮ����
���ܽ�����DO�����л����ܽ��������б�ʾˮ��DO�仯��
�ߵ��� ��ѡ��A��B��C��D��
��Ŀǰ�ƹ�ʹ������ϴ�·ۣ����ᳫ��ѧ����ʩ�û��ʣ���Ŀ���Ƿ�ֹˮ��
�����뻷����ص������
��ij�о���ѧϰ����С�������ⶨ������ˮ����Ⱦ�������
�����ǿ���С���Ա����Ҫȡ���ݲ�ͬ������ˮ������ѡ��������ص��ǣ�
�� �� ��
 �Ȱ���ˮ���л�ѧ����ʱ����ˮ�������ֲ�ͬ������ϸ���������������������ģ��������£� ��ijͬѧ�ڸõع�ҵ��ȡ��ˮ������������������ĺ��������Ĺ���������ȼ��ˮ���е�炙�Ũ�ȣ���Ӧԭ��Ϊ2K2[HgI4]+NH4Cl+4KOH==(OHg2NH2)I����ɫ��+KCl+7KI+3H2O���ñ�ɫ���ⶨ��
�Ȱ���ˮ���л�ѧ����ʱ����ˮ�������ֲ�ͬ������ϸ���������������������ģ��������£� ��ijͬѧ�ڸõع�ҵ��ȡ��ˮ������������������ĺ��������Ĺ���������ȼ��ˮ���е�炙�Ũ�ȣ���Ӧԭ��Ϊ2K2[HgI4]+NH4Cl+4KOH==(OHg2NH2)I����ɫ��+KCl+7KI+3H2O���ñ�ɫ���ⶨ���ٱ�ɫ���ܲⶨ����NH3��NH4+��Ũ�ȣ�ԭ����
������õĺ����쳣ƫ�ߣ����������������NO2����NO3��������ƫ�ߡ����ܵ�ԭ����
A�����������Ṥ�������ų��ĺ�����ķ�ˮ
B���ϳɰ��������ŷŵ����а��ķ�ˮ
C����ֽ���ų����л���ˮ�ͺϳɰ����ų������а��ķ�ˮ
��ÿ�վ�2�֣���1��C��2�� ��ֹˮ�帻Ӫ������ˮ�����ೱ�Ⱦ��ɣ�����3����������Χ����������Χ�ӣ�ũ��ӵ��ȣ�ֻҪ�д����ԣ����ظ����ɣ�����4�֣�������ɫ��dz�백�������������Ȣ�BC
��ֹˮ�帻Ӫ������ˮ�����ೱ�Ⱦ��ɣ�����3����������Χ����������Χ�ӣ�ũ��ӵ��ȣ�ֻҪ�д����ԣ����ظ����ɣ�����4�֣�������ɫ��dz�백�������������Ȣ�BC
 ��ֹˮ�帻Ӫ������ˮ�����ೱ�Ⱦ��ɣ�����3����������Χ����������Χ�ӣ�ũ��ӵ��ȣ�ֻҪ�д����ԣ����ظ����ɣ�����4�֣�������ɫ��dz�백�������������Ȣ�BC
��ֹˮ�帻Ӫ������ˮ�����ೱ�Ⱦ��ɣ�����3����������Χ����������Χ�ӣ�ũ��ӵ��ȣ�ֻҪ�д����ԣ����ظ����ɣ�����4�֣�������ɫ��dz�백�������������Ȣ�BC���������ʵ������黷����Ⱦ�뱣������ػ���֪ʶ�����кܺõ�̽���ԡ���1��ˮ����������������Ⱦ�����٣������ܽ������������Ķ����ٺ����ָ�����ˮ��DO�仯����ΪC����2���������ʵ��ŷŻ�����ˮ��ֲ�������Ӫ��������ˮ�����ೱ������֮Ϊ��ˮ�帻Ӫ����������3��ˮ�����ȾԴ��Ҫ�ǹ�ҵ���������ǻ������ŷŵķ�ˮ��ũҵ��ʹ�õĻ��ʡ�ũҩ�����о���������ˮ�ȣ�����Ӧȡ��Щ���д����Եĵ�����ˮ������4���ٱ�ɫ����Ȼ����pH��ֽ��ԭ����炙�Ũ�ȸߣ��Ӽ���Լ�������ɫ����ڰ��ĺ����쳣ƫ�ߣ�NO2����NO3��������ƫ�ߣ���ΪNO2����NO3����NH3�������������ģ���ԭ����Ȼ��BC��

��ϰ��ϵ�д�
�����Ŀ