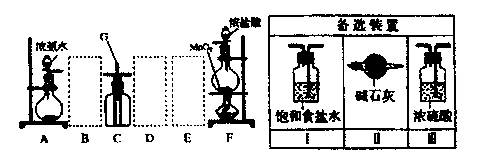
��Ŀ����
ijͬѧ��ѧУʵ���������ƿʧȥ��ǩ����Һ�����ΪA��B������ѯ��ʵ��Ա��֪��ϡ�����С�մ�(NaHCO3)��Һ��Ϊ���������ǣ���ͬѧ����������ʵ�飬����Э��������������Ľ��
(1)ȡ����A��B����Һ���ֱ��������Na2CO3���۲쵽A���д������ݣ�B��û�����Ա仯����A��Һ�з�����Ӧ�Ļ�ѧ����ʽ��____________________��
(2)ȡ����A��B����Һ���ֱ���뼸�η�̪��Һ��A��Ϊ��ɫ��BΪdz��ɫ���ֱ�����μ��������NaOH��Һ��A�е�������___________________________�������ӷ���ʽ��_________________________________��B�е�������_________________________�� �����ӷ���ʽ��___________________________________________________________��
(1)ȡ����A��B����Һ���ֱ��������Na2CO3���۲쵽A���д������ݣ�B��û�����Ա仯����A��Һ�з�����Ӧ�Ļ�ѧ����ʽ��____________________��
(2)ȡ����A��B����Һ���ֱ���뼸�η�̪��Һ��A��Ϊ��ɫ��BΪdz��ɫ���ֱ�����μ��������NaOH��Һ��A�е�������___________________________�������ӷ���ʽ��_________________________________��B�е�������_________________________�� �����ӷ���ʽ��___________________________________________________________��
��1��H2SO4 + Na2CO3��Na2SO4 + CO2��+ H2O��3�֣�
��2����Һ����ɫ��Ϊ��ɫ��1�֣� H��+ OH����H2O��3�֣�
��Һ��dz��ɫ��Ϊ��ɫ��1�֣� HCO3�� + OH����CO32��+ H2O ��3�֣�
��2����Һ����ɫ��Ϊ��ɫ��1�֣� H��+ OH����H2O��3�֣�
��Һ��dz��ɫ��Ϊ��ɫ��1�֣� HCO3�� + OH����CO32��+ H2O ��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ
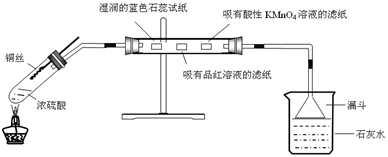
 2�����Ʊ���ijͬѧ�������һ���Ʊ�̼��Ƶķ�����������ͼΪ��
2�����Ʊ���ijͬѧ�������һ���Ʊ�̼��Ƶķ�����������ͼΪ��