��Ŀ����
������ͼ����Ũ������ͭ��Ӧ������֤������������ʡ�
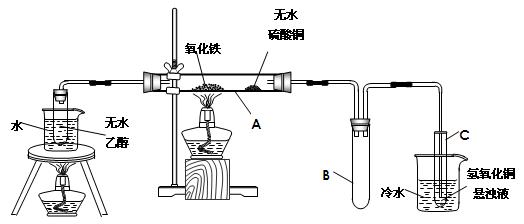
ʵ��������£����������Ͱ���ҩƷ���þƾ��Ƽ���1���Ӻ�ȥ�ƾ��ƣ���ͭ˿����Һ�����£�һ��ʱ�������ͭ˿��
��ش��������⣺
��1������Ӧ����Թܳ�־��ã��۲쵽�Թܵײ��а�ɫ������֣��м����dz��ɫҺ�壨Ũ���ᣩ���ϲ�����ɫ��Һ����ɫ����Ļ�ѧʽΪ ������������������Ũ����������� ������ţ�
������ ��ǿ������ ����ˮ�� ����ˮ��
��2��ֱ�����������ι۲쵽�������ǣ� ��
�� ��
��3��ij��ʵ����С�ձ��г���ʯ��ˮʼ��δ�۲쵽���ǣ��������ܵ�ԭ���� �������ʵ��֤���� ��
��4��ijѧ�����֣������½�ͭƬ��ʱ�����Ũ������Թ��в��ܷ⣬�������ɺ�ɫ���ʣ�Cu2S������ɫ��Һ�����������ɣ�д����������ͭƬ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
ʵ��������£����������Ͱ���ҩƷ���þƾ��Ƽ���1���Ӻ�ȥ�ƾ��ƣ���ͭ˿����Һ�����£�һ��ʱ�������ͭ˿��

��ش��������⣺
��1������Ӧ����Թܳ�־��ã��۲쵽�Թܵײ��а�ɫ������֣��м����dz��ɫҺ�壨Ũ���ᣩ���ϲ�����ɫ��Һ����ɫ����Ļ�ѧʽΪ ������������������Ũ����������� ������ţ�
������ ��ǿ������ ����ˮ�� ����ˮ��
��2��ֱ�����������ι۲쵽�������ǣ� ��
�� ��
��3��ij��ʵ����С�ձ��г���ʯ��ˮʼ��δ�۲쵽���ǣ��������ܵ�ԭ���� �������ʵ��֤���� ��
��4��ijѧ�����֣������½�ͭƬ��ʱ�����Ũ������Թ��в��ܷ⣬�������ɺ�ɫ���ʣ�Cu2S������ɫ��Һ�����������ɣ�д����������ͭƬ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
��1��CuSO4��2�֣����٢ڢۣ�2�֣�
��2����ɫʯ����ֽ��Ϊ��ɫ��2�֣�����ɫ��ֽ��Ϊ��ɫ��2�֣���
�Ϻ�ɫ��ֽ��Ϊ��ɫ��2�֣�
��3��SO2������ˮ������SO2��Ca(OH)2����������ˮ��Ca(HSO3)2��������CaSO3���ǣ�2�֣���ȡС�ձ�����Һ��������һ֧�Թ��У���������NaOH��Һ�����а�ɫ�������ɣ���֤�����������3�֣���
��4��5Cu+4H2SO4(Ũ)=Cu2S+3CuSO4+4H2O��3�֣�
��2����ɫʯ����ֽ��Ϊ��ɫ��2�֣�����ɫ��ֽ��Ϊ��ɫ��2�֣���
�Ϻ�ɫ��ֽ��Ϊ��ɫ��2�֣�
��3��SO2������ˮ������SO2��Ca(OH)2����������ˮ��Ca(HSO3)2��������CaSO3���ǣ�2�֣���ȡС�ձ�����Һ��������һ֧�Թ��У���������NaOH��Һ�����а�ɫ�������ɣ���֤�����������3�֣���
��4��5Cu+4H2SO4(Ũ)=Cu2S+3CuSO4+4H2O��3�֣�
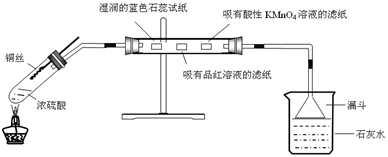
����SO2����ȡ�����ʣ�ͭ��Ũ����ķ�ӦΪ��Cu+2H2SO4(Ũ)=CuSO4+SO2��+2H2O
��1����������Ӧ�У�Ũ���Ჿ�ֱ���ԭΪSO2�����������ԣ������������ԣ�����CuSO4���ԹܵͲ��İ�ɫ����Ϊ����ͭ���壬˵��Ũ����ɽ�CuSO4��5H2O��ˮ�����յ���������Ũ�������ˮ��
��2��SO2Ϊ�����������ˮ������ǿ�SO2��H2O=H2SO3����ʹ��ɫʯ����ֽ��Ϊ��ɫ��SO2����Ư���ԣ���Ư��Ʒ����ֽ��SO2���л�ԭ�ԣ��ɽ�KMnO4��ɫ��Һ��ԭΪ��ɫ
��3���������ʱ�����ɰ�ɫ���ǣ�SO2��Ca(OH)2=CaSO3��=H2O�������д���SO2��ʯ��ˮ��Ӧʱ��Ca(OH)2��2SO2=Ca(HSO3)2��Ca(HSO3)2������ˮ�������dz���
��ʽ��Ca(HSO3)2����Ӧ���ɲ�����ˮ��CaSO3���ʿ�ȡС�ձ�����Һ��������һ֧�Թ��У���������NaOH��Һ�����а�ɫ�������ɣ���֤���������
��4�����ݻ��ϼ۵������غ㣬����ƽ�˷�Ӧ��5Cu+4H2SO4(Ũ)=Cu2S+3CuSO4+4H2O
��1����������Ӧ�У�Ũ���Ჿ�ֱ���ԭΪSO2�����������ԣ������������ԣ�����CuSO4���ԹܵͲ��İ�ɫ����Ϊ����ͭ���壬˵��Ũ����ɽ�CuSO4��5H2O��ˮ�����յ���������Ũ�������ˮ��
��2��SO2Ϊ�����������ˮ������ǿ�SO2��H2O=H2SO3����ʹ��ɫʯ����ֽ��Ϊ��ɫ��SO2����Ư���ԣ���Ư��Ʒ����ֽ��SO2���л�ԭ�ԣ��ɽ�KMnO4��ɫ��Һ��ԭΪ��ɫ
��3���������ʱ�����ɰ�ɫ���ǣ�SO2��Ca(OH)2=CaSO3��=H2O�������д���SO2��ʯ��ˮ��Ӧʱ��Ca(OH)2��2SO2=Ca(HSO3)2��Ca(HSO3)2������ˮ�������dz���
��ʽ��Ca(HSO3)2����Ӧ���ɲ�����ˮ��CaSO3���ʿ�ȡС�ձ�����Һ��������һ֧�Թ��У���������NaOH��Һ�����а�ɫ�������ɣ���֤���������
��4�����ݻ��ϼ۵������غ㣬����ƽ�˷�Ӧ��5Cu+4H2SO4(Ũ)=Cu2S+3CuSO4+4H2O

��ϰ��ϵ�д�
�����Ŀ

 H)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣�
H)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣� CO2����2H2O��2SO2��
CO2����2H2O��2SO2��
 ��˳���������������ҵķ����ǣ���װ�õı�ţ���
��˳���������������ҵķ����ǣ���װ�õı�ţ��� �����ܡ���__________��__________��__________��
�����ܡ���__________��__________��__________�� ��������Ϊbg����a��b��ʾCO2��SO2�����ʵ���������������������
��������Ϊbg����a��b��ʾCO2��SO2�����ʵ���������������������