��Ŀ����
��Уij��ѧС�������ʵ�����ŨH2SO4��ľ̿��Ӧ�����в���:
(1)д��ŨH2SO4��ľ̿��Ӧ�Ļ�ѧ����ʽ
(2)�������ͼ��ѡ����������������ظ�ʹ�ã����һ��װ�ð�����С��ʵ�����ǵ�Ŀ��.���ṩŨH2SO4��ľ̿������KMnO4��Һ����ˮ����ͭ�������̡�Һ�Լ���ѡ�������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ���ȥ��
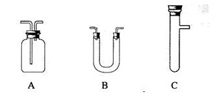
����ѡ������������˳�������������������±�(���Բ�����,Ҳ���Բ���)����д����������Ӧ���Լ������Ƽ������á�
(1)д��ŨH2SO4��ľ̿��Ӧ�Ļ�ѧ����ʽ
(2)�������ͼ��ѡ����������������ظ�ʹ�ã����һ��װ�ð�����С��ʵ�����ǵ�Ŀ��.���ṩŨH2SO4��ľ̿������KMnO4��Һ����ˮ����ͭ�������̡�Һ�Լ���ѡ�������Ӻ̶������õIJ����ܡ����ܡ����С�����̨������װ�õȾ���ȥ��

����ѡ������������˳�������������������±�(���Բ�����,Ҳ���Բ���)����д����������Ӧ���Լ������Ƽ������á�
| ѡ�õ����� ������ĸ�� | ������Լ� | ���� |
| (1) | ��ˮ����ͭ | |
| (2) A | | |
| (3) | ����KMnO4��Һ | |
| (4) | | ����SO2�Ƿ��ѳ��� |
| (5) A | | |
��12�֣���1��2 H2SO4 (Ũ) + C 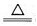 CO2��+ 2 H2O + 2SO2��
CO2��+ 2 H2O + 2SO2��
��2����ÿ��2�֣���һ����һ�֣����֣�
 CO2��+ 2 H2O + 2SO2��
CO2��+ 2 H2O + 2SO2����2����ÿ��2�֣���һ����һ�֣����֣�
| ѡ�õ�����������ĸ�� | ������Լ� | ���� |
| (1)B (2)A (3)A (4)A (5)A | ��ˮ����ͭ Ʒ�� ����KMnO4��Һ Ʒ�� ����ʯ��ˮ | ����ˮ������ ����SO2������ ��ȥSO2 ����SO2�Ƿ��ѳ��� ����CO2������ |
ľ̿��Ũ��������2�����壬CO2/SO2������Ҫ�ȼ���SO2���ټ���CO2�����Ҽ���ǰҪ��ȥSO2����ֹ����CO2�ļ��顣����ˮ�ļ���Ҫ��ǰ�棬��Ϊ���������������ˮ�֡�

��ϰ��ϵ�д�
�����Ŀ
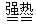 Fe2O3+SO2��+ SO3��+ 14H2O
Fe2O3+SO2��+ SO3��+ 14H2O o
o

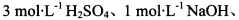 ����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
����KMnO4��Һ������NaHCO3��Һ��Ʒ����Һ������ʯ��ˮ
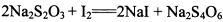 ��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������
��Ϊ�ⶨ���Ƶþ���Ĵ��ȣ���С���Ե�����ָʾ������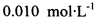 �ĵ�ˮ���ж��ȡ���ζ������
�ĵ�ˮ���ж��ȡ���ζ������ �ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��
�ĺ���ԼΪ102%���������Լ��������������������ý������ܵ�ԭ����______________��