��Ŀ����
����Ŀ���Ȼ�������![]() ��һ����Ҫ�Ĺ�ҵԭ�ϣ���ҵ�ϳ��ø�����
��һ����Ҫ�Ĺ�ҵԭ�ϣ���ҵ�ϳ��ø�����![]() ���Ʊ���ʵ�������Ժ췯��
���Ʊ���ʵ�������Ժ췯��![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() ���������£�
���������£�
��֪����![]() ��
��
��![]() ���������ѣ�������ˮ���Ҵ�����ˮ�⣻
���������ѣ�������ˮ���Ҵ�����ˮ�⣻
�ۼ״�����ɫҺ�壬�ж����е�64.7����ȼ��
��ش�
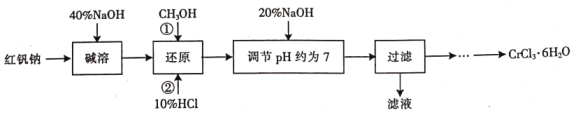
(1)���ܵ�Ŀ����________________��
(2)��ԭʱ�ȼ������![]() �ټ���10%HCl������
�ټ���10%HCl������![]() ���÷�Ӧ�����ӷ���ʽΪ________________��
���÷�Ӧ�����ӷ���ʽΪ________________��
(3)��������Һ�з����![]() ѭ��ʹ�á�
ѭ��ʹ�á�
�������й�����ʵ��˵����ȷ����_________(�����)��
A.����ʼ��Ӧ�ȼ�����ͨ����ˮ
B.ͨ������ɳ�ȥҺ�����ѻӷ��ӷ�������
C.���¶ȼƲ�����Һ�У�����ռ����е�ƫ�ߵ�����
D.������е���ߵijɷ�ʱ��Ӧ�ÿ�������
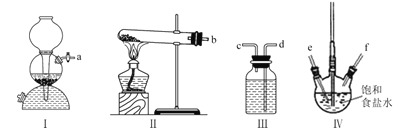
��ʵ���������ͼװ�ý�������װ���д��ڵĴ�����_________��
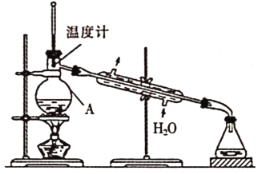
���ڸ������װ���У�������A�м����ʯ���ټ�����Һ�������ռ��������![]() ���е�ͬѧ��Ϊ���ù�������Ҫ����CaO����ֹˮ��
���е�ͬѧ��Ϊ���ù�������Ҫ����CaO����ֹˮ��![]() һ������������Ϊ�Ƿ��б�Ҫ��˵�����ɣ�_______��
һ������������Ϊ�Ƿ��б�Ҫ��˵�����ɣ�_______��
(4)�벹�������ɹ��˺�õ��Ĺ���![]() �Ʊ�
�Ʊ�![]() ��ʵ�鷽��(�ɹ�ѡ����Լ������ᡢ���ᡢ����ˮ���Ҵ�������)�������˺����ù���__________��__________����ȴ�ᾧ�����ˣ�__________�����¸���õ�
��ʵ�鷽��(�ɹ�ѡ����Լ������ᡢ���ᡢ����ˮ���Ҵ�������)�������˺����ù���__________��__________����ȴ�ᾧ�����ˣ�__________�����¸���õ�![]() ��
��
���𰸡����췯��(��Cr2O72-)ת��Ϊ������(��CrO42-) 10H++2CrO42-+CH3OH=2Cr3++7H2O+CO2�� BD ��������ȡ���ƿ��ϵ��� û�б�Ҫ��CH3OH���뷴Ӧ����ϵ����ˮ ��ȫ�ܽ��ڹ����������� ����Ũ�� ������ϴ�ӹ���2��3��
��������
��40%NaOH���췯��(Na2Cr2O7)ת��Ϊ������(Na2CrO4)������CH3OH��������(Na2CrO4)��ԭΪCrCl3��Һ����20%NaOHʹCr3+����ΪCr(OH)3�����ˣ������˺����ù����������ܽ⣬���CrCl3��HCl��Һ��������Ũ������ȴ�ᾧ�����˲�������ϴ��2��3�Σ����¸������CrCl36H2O���塣
(1)����ʹCr2O72-+H2O2CrO42-+2H+��ƽ�������ƶ������췯��(��Cr2O72-)ת��Ϊ������(��CrO42-)��������40%NaOH���˹���̫�࣬�����Ӹ�������ỹԭ���ʴ�Ϊ�����췯��(��Cr2O72-)ת��Ϊ������(��CrO42-)��
(2)CH3OH��������(Na2CrO4)��ԭΪCrCl3��Һ����ӦΪ10H++2CrO42-+CH3OH=2Cr3++7H2O+CO2�����ʴ�Ϊ��10H++2CrO42-+CH3OH=2Cr3++7H2O+CO2����
(3)��A��ʵ�鿪ʼʱ��Ϊ��ֹ���������ѣ�Ӧ�Ƚ�ͨ����ˮ���ٵ�ȼ�ƾ��Ƽ���������ƿ����A����
B��CH3OH���ѻӷ��ӷ������ʷе�ߣ�CH3OH�ķе�ͣ�������������Գ�ȥCH3OH��Һ�����ѻӷ��ӷ������ʣ���B��ȷ��
C������ʱ�ⶨ��ֵ��¶ȣ��¶ȼƲ���������ƿ��֧�ܿڴ�����C����
D��ˮ�������¶Ƚϵͣ��²���������ܻ�ը�ѣ�������е���ߵijɷ�ʱ��Ӧ�ÿ�����������D��ȷ��
�ʴ�Ϊ��BD��
�ڼ״��е�Ϊ64.7������ȼ������ˮԡ���ȣ�������������ƿ��ϵ��գ�����������ռ����ʴ�Ϊ����������ȡ���ƿ��ϵ��գ�
��CH3OH���뷴Ӧ����ϵ��ˮ��Һ�����û�б�Ҫ����CaO���ʴ�Ϊ��û�б�Ҫ��CH3OH���뷴Ӧ����ϵ����ˮ��
(4)��֪��CrCl36H2O���������ѣ����˺�����Cr(OH)3�ù����������ܽ⣬���CrCl3��HCl��Һ��������Ũ������ȴ�ᾧ�����˲�������ϴ��2��3�Σ�����CrCl36H2O���壻�ʴ�Ϊ����ȫ�ܽ��ڹ����������У�����Ũ����������ϴ�ӹ���2��3�Ρ�

����Ŀ����������������Ӧ����㷺�Ľ����������������ܹ�ע����֪��
��![]()
![]()
��![]()
![]()
�۶���![]() �����еĻ�ѧ����Ҫ����1076kJ������������
�����еĻ�ѧ����Ҫ����1076kJ������������![]() �����еĻ�ѧ����Ҫ����1490kJ��������
�����еĻ�ѧ����Ҫ����1490kJ��������
��ش�
(1)����![]() �����л�ѧ����Ҫ���յ�����Ϊ________kJ��
�����л�ѧ����Ҫ���յ�����Ϊ________kJ��
(2)T1��ʱ�����ܱ������м���һ������![]() ��C��������Ӧ�٣��ﵽƽ���
��C��������Ӧ�٣��ﵽƽ���![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() �������������䣬��С����������ٴδﵽƽ��ʱ��
�������������䣬��С����������ٴδﵽƽ��ʱ��![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ����a________b(ѡ����>����<������=��)��
����a________b(ѡ����>����<������=��)��
(3)��ʼ�¶Ⱦ�ΪT2��ʱ�����ݻ�Ϊ10L�����������ܱ������У��ֱ����һ������![]() ��CO������Ӧ�ڣ����������������ʾ��
��CO������Ӧ�ڣ����������������ʾ��
��� | ���� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ƽ�ⳣ��(K) | |
|
|
| |||
1 | ���� | 0.5 | 1.5 | 0.8 K1 | |
2 | ���� | 2 | 3 | M | K2 |
3 | ���� | 1 | 1.5 | n | K3 |
��T2��ʱ������1�з�Ӧ��ƽ�ⳣ��K1=_______��
������2�У�5min�ﵽƽ�⣬��0~5min��![]() ��ʾ�÷�Ӧ������
��ʾ�÷�Ӧ������![]() ___________��
___________��
�۶������������еķ�Ӧ������˵����ȷ����(����ĸ)_________��
A.![]() B.����1������2��
B.����1������2��![]() ��ƽ��ת����
��ƽ��ת����![]()
C.![]() D.ƽ��ʱ����ѹǿ��
D.ƽ��ʱ����ѹǿ��![]()
(4)һ�������£����ѹ�ܱ������г���0.5mol ![]() ��1.0mol CO��������Ӧ�ڣ�CO��
��1.0mol CO��������Ӧ�ڣ�CO��![]() �����ʵ���Ũ��(c)��ʱ��(t)�Ĺ�ϵ��ͼ��ʾ��
�����ʵ���Ũ��(c)��ʱ��(t)�Ĺ�ϵ��ͼ��ʾ��

��6minʱ�ı���������Ϊ________________��
������6minʱ�����½������������10L������ͼ�л���6~10min![]() �����ʵ���Ũ����ʱ��仯������_________��
�����ʵ���Ũ����ʱ��仯������_________��