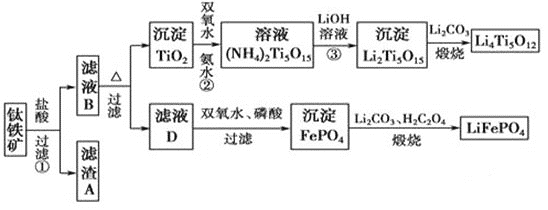
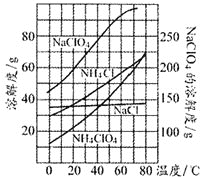
��Ŀ����
����Ŀ�����º����£���2mol����A��2mol����Bͨ���Ϊ2L���ܱ������У��������·�Ӧ��2A(g)+B(g)![]() xC(g)+2D(s)��2min��Ӧ�ﵽƽ��̬����ʱʣ��1.2molB�������C��ŨΪ1.2mol��L-1��
xC(g)+2D(s)��2min��Ӧ�ﵽƽ��̬����ʱʣ��1.2molB�������C��ŨΪ1.2mol��L-1��
��1���ӷ�Ӧ��ʼ���ﵽƽ��״̬������C��ƽ��Ӧ����Ϊ_��
��2��x=__��
��3�����и������Ϊ�÷�Ӧ�ﵽƽ��״̬��־��_������ţ���
A.ѹǿ���ٱ仯
B.�����ܶȲ��ٱ仯
C.�����ƽ����Է����������ٱ仯
D.A������������B����������֮��Ϊ
���𰸡�0.6mol��L-1��min-1 3 BC
��������
��1���ӷ�Ӧ��ʼ���ﵽƽ��״̬������C��ƽ����Ӧ����![]() ���ʴ�Ϊ��0.6mol��L-1��min-1��
���ʴ�Ϊ��0.6mol��L-1��min-1��
��2����2min�ڣ�C�����ʵ���������![]() ��B�����ʵ���������
��B�����ʵ���������![]() ��
��![]() ���������ʷ�Ӧʱ�ǰ��շ���ʽ�л�ѧ��������ϵ���еģ�����1:3=1:x����
���������ʷ�Ӧʱ�ǰ��շ���ʽ�л�ѧ��������ϵ���еģ�����1:3=1:x����![]() ���ʴ�Ϊ��3��
���ʴ�Ϊ��3��
��3��A���÷�Ӧ���������������ķ�Ӧ���κ�ʱ�������ѹǿʼ�ղ��䣬A����
B���÷�Ӧ�Ƿ�Ӧǰ������������ı�ķ�Ӧ�������������ݻ����䣬�����������ܶȲ��ٱ仯��˵��������������ٸı䣬˵����Ӧ�Ѵﵽƽ��״̬��B��ȷ��
C���÷�Ӧ�Ƿ�Ӧǰ���������ʵ�������ķ�Ӧ���������ƽ����Է����������ٱ仯����ô������������ٱ仯����Ӧ�ﵽƽ��״̬��C��ȷ��
D��A������������B����������֮��Ϊ2��1�������Ķ�������Ӧ���ʣ������жϷ�Ӧ�Ƿ�ﵽƽ��״̬��D����
����������BC���жϷ�Ӧ�ﵽƽ��״̬���ʴ�Ϊ��BC��

����Ŀ����������������һ������ȱ����ƶѪ�İ�ȫ��Ч�����Ƽ������������ʵ���pH��������![]() ����Ӧ�ø�����������
����Ӧ�ø�����������
��֪��
�������� | ��ѧʽ | ��Է������� | ���볣��(25��) |
������ |
| 116 |
|
̼�� |
|
| |
���������� | FeC4H2O4 | 170 |
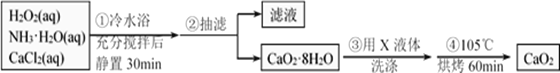
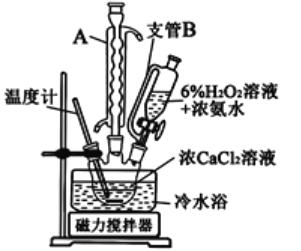
i.�Ʊ�������������
�ٽ�����������100mL�ձ�A�У�����ˮ����
�ڼ���![]() ��Һ10mLʹ��ҺpHΪ6.5-6.7������������Һ����100mL����B��
��Һ10mLʹ��ҺpHΪ6.5-6.7������������Һ����100mL����B��
�۰�װ�û���װ��C���������С�Ȼ��ͨ����ѹ��Һ©��D��������![]() ��Һ30mL
��Һ30mL
��ά�ַ�Ӧ�¶�100�棬��ֽ���1.5Сʱ����ȴ����ѹ���ˣ���ˮϴ�ӳ���
�����ˮԡ������غ�(����)ɫ��ĩ����¼����
��ش��������⣺
(1)��ʵ���漰������Ҫ����������ϸ���ѡ����BΪ__________(����ĸ)��CΪ___________(������)��

(2)��֪��Ӧ�����з�Ӧ��![]() �����ᰴǡ����ȫ��Ӧ�ı���������ʼͶ�ϣ�д��������м���
�����ᰴǡ����ȫ��Ӧ�ı���������ʼͶ�ϣ�д��������м�����Һ����pH��Ŀ����______________________________________________��������
![]() ��Һ��������ҺpHƫ�ߣ����Ƶò�Ʒ�Ĵ��Ȼ�_____________(����ƫ������ƫ����������Ӱ����)��
��Һ��������ҺpHƫ�ߣ����Ƶò�Ʒ�Ĵ��Ȼ�_____________(����ƫ������ƫ����������Ӱ����)��
(3)����![]() ��Һ����Ϊ��ȡ
��Һ����Ϊ��ȡ![]() ���壬����30mL����й�����ˮ�У���������й�����ˮ��ԭ����____________________________________________________________________________��
���壬����30mL����й�����ˮ�У���������й�����ˮ��ԭ����____________________________________________________________________________��
(4)�������ϴ�ӵ�Ŀ����Ҫ��Ϊ�˳�ȥ��___________���ӣ���������Ƿ�ϴ���ķ����ǣ�_____________��
ii.�����Ʒ�IJ��ʣ�
(5)���ⶨ����Ʒ�и����������Ĵ���Ϊ76.5%����5.80g������Ϊԭ�Ϸ�Ӧ�Ƶò�Ʒ8.10g�������������IJ���Ϊ_____________%��(����С�����һλ)