��Ŀ����
��֪����MnO2+4HCl��2KMnO4+16HCl�T�T2KCl+2MnCl2+5Cl2��+8H2O����4HCl+O2![]() 2Cl2+2H2O�����ʣ�1������Ԫ�ػ��ϼ۱仯����������Ӧ�Ĺ�ͬ����________________����������������������ǿ������˳����_________________________________����2���ڷ�Ӧ���У���������________________________________������������________________________________�����������뻹ԭ������Ӹ���֮��Ϊ________________________________����Ӧ�б�������δ��������HCl����֮��Ϊ________________________������36.5gHCl��Ӧ�������Cl2����Ϊ________________________��
2Cl2+2H2O�����ʣ�1������Ԫ�ػ��ϼ۱仯����������Ӧ�Ĺ�ͬ����________________����������������������ǿ������˳����_________________________________����2���ڷ�Ӧ���У���������________________________________������������________________________________�����������뻹ԭ������Ӹ���֮��Ϊ________________________________����Ӧ�б�������δ��������HCl����֮��Ϊ________________________������36.5gHCl��Ӧ�������Cl2����Ϊ________________________��
�𰸣�
������
������
| ��1����Ԫ����-1�����ߵ�0�ۣ�KMnO4>MnO2>O2
��2��KMnO4��Cl2��5��2��5��3��22.1875g ����Խ�ߵģ���������Խ����
|

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
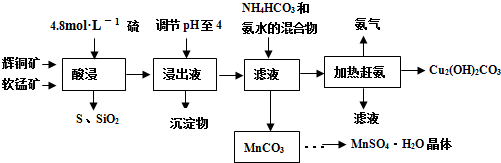
 ��֪��MnO2+4HCl��Ũ��
��֪��MnO2+4HCl��Ũ��