��Ŀ����
����Ŀ��A��B��C��D����Ԫ�ص������������Ϊ+1��+4��+5��+7����˵������B��A��C��D�Ĵ���������֪Bԭ�ӵĴ���������Ϊ2��A��C��Dԭ�ӵĴ�����������Ϊ8��Aԭ�Ӻ����������������20���Իش�
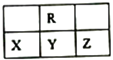
(1)��Ԫ�ط���Ϊ��A________B________C________D_______
(2)д��C��D��Ӧ���⻯���ȶ��Դ�С����Ϊ��___________���û�ѧʽ��գ���A������������Ӧ��ˮ������D���⻯�ﷴӦ�����ӷ���ʽΪ__________________��
(3)д���������ʵĵ���ʽ��AD_______________��BD4________________��
���𰸡� Na C P Cl PH3��HCl OH-+H+==H2O 
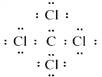
�����������⿼��Ԫ�����ڱ����ƶϡ�(1)��֪Bԭ�ӵĴ���������Ϊ2����BԪ�ص��������Ϊ+4���˵��������B��A��C��D�Ĵ�������BΪCԪ�أ�A��C��Dԭ�ӵĴ�����������Ϊ8��Aԭ�Ӻ����������������20����AԪ�ص��������Ϊ+1��AΪNa��CԪ�ص��������Ϊ+5��CΪP��DԪ�ص��������Ϊ+7��DΪCl��(2)�ǽ�����Խǿ�����⻯��Խ�ȶ�����C��D��Ӧ���⻯���ȶ��Դ�С����ΪPH3��HCl��A������������Ӧ��ˮ����ΪNaOH��D���⻯��ΪHCl���䷴Ӧ�����ӷ���ʽΪ��OH-+H+==H2O��(3) ������ADΪ�Ȼ��ƣ��������ӻ���������ʽΪ![]() ��BD4ΪCCl4�����ڹ��ۻ���������ʽΪ��
��BD4ΪCCl4�����ڹ��ۻ���������ʽΪ��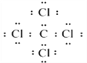 ��
��

 ����С״Ԫ��������������ϵ�д�
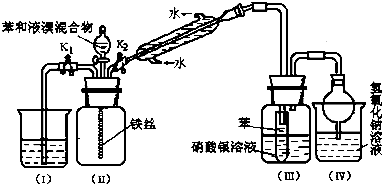
����С״Ԫ��������������ϵ�д�����Ŀ��ijͬѧ�����ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ�� 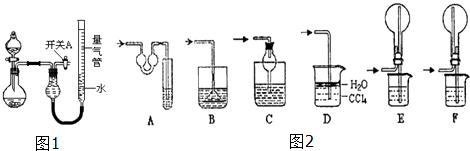
ʵ�� | ҩƷ | ��ȡ���� | �������е�Һ�� |
�� | Cu��ϡHNO3 | H2O | |
�� | NaOH���塢Ũ��ˮ | NH3 | |
�� | Na2CO3���塢ϡH2SO4 | CO2 | |
�� | þ���Ͻ�NaOH��Һ�������� | H2 | H2O |
��ش��������⣺
��1��������μ���װ�õ������ԣ� ��
��2����ͬѧ��Ϊʵ��ٿ�ͨ���ռ�����NO������������̽��Cu��Ʒ�Ĵ��ȣ�����Ϊ�Ƿ���У������ԭ�� ��
��3��ʵ�����ʣ���NH3�����մ��������¸���β������װ���У��ʺ�������NH3 �� �����ܷ�ֹ��������
��4��ʵ����У��������е�Һ������� ��
A.H2O
B.CCl4
C.����Na2CO3��Һ
D.����NaHCO3��Һ
��5����ʵ��Ӧ�������ܶ�ζ���������ʱӦע�⣺ �ٻָ������£��� �� �������밼Һ����ʹ���ƽ��
��6��ʵ��ܻ���������ݣ���������������ѻ���ɱ�״����
��� | þ���Ͻ����� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
�� | 1.0g | 10.0mL | 346.3mL |
�� | 1.0g | 10.0mL | 335.0mL |
�� | 1.0g | 10.0mL | 345.7mL |
���������������ݼ���þ���Ͻ��������������� ��
����Ŀ��Na��Cu��Fe��Cl��O��N�dz�����6��Ԫ�أ�
��1��Feλ��Ԫ�����ڱ������ڵ��壻O�Ļ�̬ԭ�Ӻ�������δ�ɶԵ��ӣ�Cu�Ļ�̬ԭ�ӵ����Ų�ʽΪ ��
��2���á�����������գ�
��һ������ | ԭ�Ӱ뾶 | �ȶ��� |
NO | CNa | NH3H2O |
��3��Һ̬N2H4��Һ̬N2O4��Ӧ������̬ˮ����25�桢101kPa�£���֪�÷�Ӧÿ����1mol N2H4�ų�519.85kJ���������÷�Ӧ���Ȼ�ѧ��Ӧ����ʽ�ǣ�
��4�����ϵĵ����������õ��Ʊ�N2H4�ķ�����NaClO��Һ����������NH3 �� д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ ��