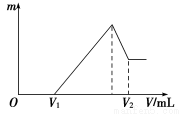
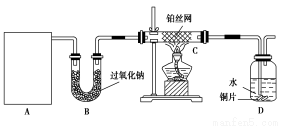��Ŀ����
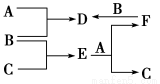
A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬C
Ϊ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ(����ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ)��
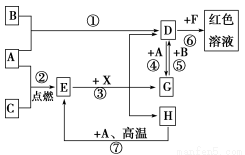
(1)д����ѧʽ��A________��D________��E________��X________��
(2)�ڷ�Ӧ�������У�������������ԭ��Ӧ����________(����)��
(3)��Ӧ�������ӷ���ʽΪ��_______________________________________��
(4)��Ӧ���Ļ�ѧ����ʽΪ_________________________________________��
�÷�Ӧ��ÿ����0.3 mol��A����ת�Ƶ���______mol��
(5)д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽ��_________________________
(1)Fe��FeBr3��Fe3O4��HBr��(2)�ۢ�
(3)Fe3����3SCN��=Fe(SCN)3
(4)3Fe��4H2O(g) Fe3O4��4H2��0.8
Fe3O4��4H2��0.8
(5)Fe3����3HCO3��=Fe(OH)3����3CO2��
�������������ϢA��B��CΪ��ѧ�������ʣ�����һ��Ϊ������BΪҺ�壬������ȷ��BΪBr2��AΪ���壬��ȷ��AΪ����������D��G��ת����ϵ���Ʋ�A���ڱ�۽���Ԫ�أ���һ��ȷ��AΪFe��EΪ��ɫ���壬����һ������ǿ�ᷴӦ�������������ʣ��²�EΪFe3O4��HΪH2O��DΪ FeBr3��GΪFeBr2��XΪHBr��FΪ���軯�

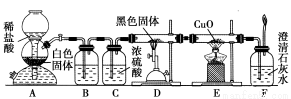
��������ɸ�������ɺ����ʽ��з��ࣺ
(1)��ͼ��ʾ�����ʷ����������________
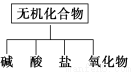
(2)��Na��K��H��O��C��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��ڢۢ��ĺ��档
������� | �� | �� | �� | ������ |
��ѧʽ | HCl��____ | ��___��Ba(OH)2 | ��Na2CO3��____ | ��CO2��Na2O2 |
(3)д����ת��Ϊ���Ļ�ѧ����ʽ_____________________________
(4)���������������ΪO2��Դ�ķ�Ӧԭ��Ϊ��___________________________
(5)ʵ�����Ʊ�������________��________��Ӧ�����������ķ�����____________