��Ŀ����
����Ŀ���ʰ���п��һ������ʳƷӪ��ǿ����������ZnO��ʰ��� �Ʊ���
�Ʊ���
(1)Zn2+��̬��������Ų�ʽΪ__________________��
(2)�ʰ��������̼ԭ�ӹ�����ӻ�������________________��1mol �ʰ�������к��ЦҼ�����ĿΪ________________��
(3)������п����Ϊԭ�ϣ���ȡп���йط�ӦΪ��ZnO+2NH3+2NH4+= [Zn(NH3)4]2++H2O����NH4+��Ϊ�ȵ������������Ϊ_____��[Zn(NH3)4]2+�Ľṹ����ʾ��ͼ��ʾΪ______________��
(4) ��п�����Ҫ�ɷ���һ��п������侧���ṹ��ͼ��ʾ���仯ѧʽΪ___________��

���𰸡�1s22s22p63S23p63d10��[Ar]3d10 sp3��sp2 9��6.02��1023(��9NA) BH4- 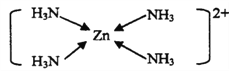 ZnS
ZnS
��������
��1����������Ų�ʽ����д��Znλ�ڵ�������IIB�壬���Zn2���ĺ�������Ų�ʽΪ��1s22s22p63S23p63d10��[Ar]3d10 ����2�������ӻ����͵��жϡ���ѧ����Ŀ���жϣ��ʰ����У�CH2����Cԭ���ӻ�����Ϊsp3���Ȼ���̼ԭ���ӻ�����Ϊsp2���ɼ�ԭ�Ӽ�ֻ���γ�һ���Ҽ������1mol�ʰ����ЦҼ�����Ŀ��9NA��5.418��1024����3������ȵ�������жϣ��Լ��������д����NH4����Ϊ�ȵ��������������BH4����Zn2������λ��Ϊ4��Zn2���ṩ�չ����NH3��N�ṩ�µ��Ӷԣ����ʾ��ͼΪ ����4�����龧���ļ��㣬Sλ�ڶ�����ڲ�������Ϊ8��1/8��1=2��Znλ�����Ϻ��ڲ�������Ϊ4��1/4��1=2����ѧʽΪZnS��
����4�����龧���ļ��㣬Sλ�ڶ�����ڲ�������Ϊ8��1/8��1=2��Znλ�����Ϻ��ڲ�������Ϊ4��1/4��1=2����ѧʽΪZnS��



