��Ŀ����
��12�֣���һ����������ܺ����Ȼ������������ơ����������Ȼ�ͭ�������ơ���ʯ�Һ��Ȼ�淋�7�����ʡ������������ˮ�ð�ɫ��������ɫ��Һ����ɫ���������ڰ�ˮ�С���ɫ��Һ��ǿ��ȷų��������ʹ��̪��졣��ɫ��ҺҲ��ʹ���Ը��������Һ��ɫ����ɫ��Һ����ʱ�ɷų�������
��֪��Ksp(AgNO2)=6.0��10-4��Ksp(AgCl)=1.56��10-10
�ش��������⣨�û�ѧ���Żش𣩣�
��1���ù��������п϶����ڵ�������________________���϶������ڵ�������_______________��
��2�����������ˮʱ���õİ�ɫ�����п϶���________________���ó������ڰ�ˮ�����ӷ���ʽΪ_______________________________________��
��3����ɫ��Һ�У�ʹ���Ը��������ɫ��������___________________��
��4��д����ɫ��Һ����ʱ�ų������Ļ�ѧ����ʽ__________________��
��֪��Ksp(AgNO2)=6.0��10-4��Ksp(AgCl)=1.56��10-10
�ش��������⣨�û�ѧ���Żش𣩣�
��1���ù��������п϶����ڵ�������________________���϶������ڵ�������_______________��
��2�����������ˮʱ���õİ�ɫ�����п϶���________________���ó������ڰ�ˮ�����ӷ���ʽΪ_______________________________________��
��3����ɫ��Һ�У�ʹ���Ը��������ɫ��������___________________��
��4��д����ɫ��Һ����ʱ�ų������Ļ�ѧ����ʽ__________________��
��1��NH4Cl��NaNO2��AgNO3 FeCl3��CuCl2��Ca(OH)2
��2��AgCl AgCl+2NH3?H2O=Ag(NH3)2Cl+2H2O ��3��NaNO2
��4��NaNO2+NH4Cl N2��+NaCl+2H2O
N2��+NaCl+2H2O
��2��AgCl AgCl+2NH3?H2O=Ag(NH3)2Cl+2H2O ��3��NaNO2
��4��NaNO2+NH4Cl
 N2��+NaCl+2H2O
N2��+NaCl+2H2O��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ����
����

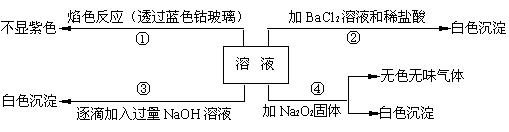 ��1����ԭ��Һ��һ�������ڵ������� ��
��1����ԭ��Һ��һ�������ڵ������� ��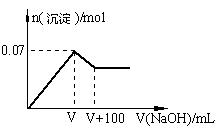

 ����Һ�������Լ�Ҳ�ܼ���������
����Һ�������Լ�Ҳ�ܼ���������