��Ŀ����
��6�֣�����������һ��ʹ�ù㷺�Ļ�ѧ�Լ���ij����С��ͨ������ʵ��ⶨij������Ba(OH)2��nH2O�ĺ�����
��1����ȡ3.50g������������ˮ���100mL��Һ������ȡ��10.0mL��Һ����ƿ�У���0.100mol/LHCl��Һ�кͣ�����������20.0mL�����ʲ����ᷴӦ�������������������������ʵ�����
��2����ȡ5.25g����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�����Ϊ3.09g����Ba(OH)2��nH2O�е�nֵ��
��1����ȡ3.50g������������ˮ���100mL��Һ������ȡ��10.0mL��Һ����ƿ�У���0.100mol/LHCl��Һ�кͣ�����������20.0mL�����ʲ����ᷴӦ�������������������������ʵ�����
��2����ȡ5.25g����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�����Ϊ3.09g����Ba(OH)2��nH2O�е�nֵ��
��6�֣���1��0.01mol (2) 8
��

��ϰ��ϵ�д�
�����Ŀ
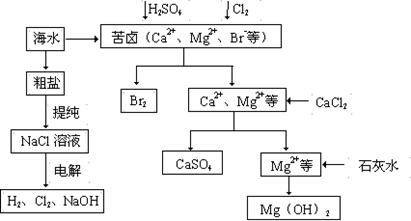
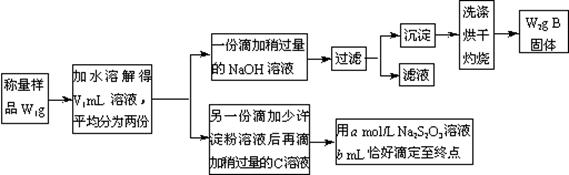
 __��
__��