��Ŀ����
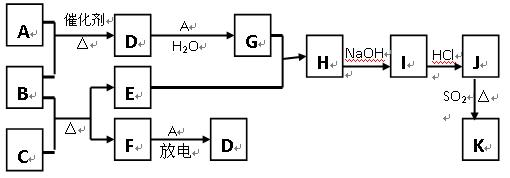
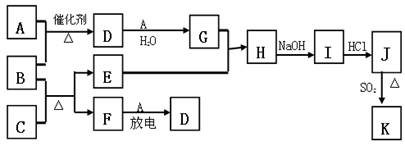
��10�֣���ͼ�漰����������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�ء���֪��A��FΪ��ɫ���嵥�ʣ�BΪ���д̼�����ζ�����壬CΪ��ɫ�����EΪ��ɫ�������ʣ����ַ�Ӧ�IJ���δ�г�������ش��������⣺
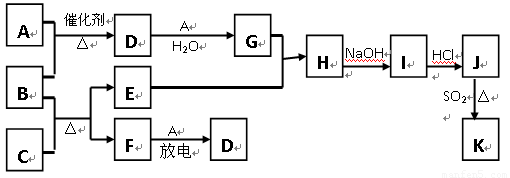
��1��B�ĵ���ʽΪ ��
��2��д��B��C��Ӧ�Ļ�ѧ����ʽ ��
��3��д��E��G��ϡ��Һ�����ӷ���ʽ��д������ת����Ŀ�� �� ��
��4������β���г�����D��CO�������ڴ��������¿��Դ�ת��Ϊ���ֶԿ�������Ⱦ�����ʣ���֪��F(g) + A(g) = 2D (g) ����H = +180.5KJ/mol
2C (s)+ O2 (g)= 2CO(g) ����H = -221.0 KJ/mol
C (s)+ O2(g) = CO2(g)����H = -393.5 KJ/mol
������β��ת�����Ȼ�ѧ����ʽΪ�� ��
���𰸡�
��1��NH3��(��)
��2��3CuO + 2NH3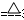 3Cu + N2 +3H2O(����)
3Cu + N2 +3H2O(����)
��3�� 3Cu +8H+ + 2NO3- = 3Cu2+ + 2NO + 4H2O 6e-
��4��2NO(g)+2CO(g)  N2(g)+2CO2(g)����H= ��746.5KJ/mol
N2(g)+2CO2(g)����H= ��746.5KJ/mol
��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ