��Ŀ����
ij�ᾧˮ���ﺬ�����������Ӻ�һ�������ӣ���ȡ����������Ϊ45.3g�ĸýᾧˮ����ֱ��Ƴ���Һ��������һ����μ���NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����������ݳ����������д̼�����ζ����ʹʪ��ĺ�ɫʯ����ֽ���������Ⱥƿ��ռ���2.24L�����壨��״����������ɫ�������ٲ�������ʧ��
��1����ʹʪ��ĺ�ɫʯ����ֽ�������д̼�����ζ�������� ��˵��ԭ�ᾧˮ�������� ����
��2������NaOH��Һ���룬��ɫ��������������ɫ�������ٲ�������ʧ��˵��ԭ�ᾧˮ�������� ������һ����μ���Ba��OH��2��Һ����ʼ�������ƣ����������а�ɫ���������ˣ���ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����46.6g��
��3��46.6�İ�ɫ����Ϊ ��˵��ԭ�ᾧˮ�������� ���ӣ�
��4���������ƶϽ�ϵ���غ㣬ԭ�ᾧˮ�������еĸ���������֮��Ϊ�� ���ýᾧˮ����Ļ�ѧʽΪ ��
��5���øýᾧˮ�����Ƴ���Һ��Ba��OH��2��Һ��Ӧ
�������ó����������ʵ��������Ӧ�����ӷ���ʽΪ�� ��
����������Ba��OH��2��Һ��Ӧ����Ӧ�����ӷ���ʽΪ�� ��
���������Ba��OH��2��Һ��Ӧ����Ӧ�����ӷ���ʽΪ�� ��
��1����ʹʪ��ĺ�ɫʯ����ֽ�������д̼�����ζ��������
��2������NaOH��Һ���룬��ɫ��������������ɫ�������ٲ�������ʧ��˵��ԭ�ᾧˮ��������
��3��46.6�İ�ɫ����Ϊ
��4���������ƶϽ�ϵ���غ㣬ԭ�ᾧˮ�������еĸ���������֮��Ϊ��
��5���øýᾧˮ�����Ƴ���Һ��Ba��OH��2��Һ��Ӧ
�������ó����������ʵ��������Ӧ�����ӷ���ʽΪ��
����������Ba��OH��2��Һ��Ӧ����Ӧ�����ӷ���ʽΪ��
���������Ba��OH��2��Һ��Ӧ����Ӧ�����ӷ���ʽΪ��
���㣺�������ӵļ��鷽��,���ӷ���ʽ����д,̽�����ʵ���ɻ�������ʵĺ���
ר�⣺���ӷ�Ӧר��
��������1����ʹʪ��ĺ�ɫʯ����ֽ�����������ǰ�����֤��笠����ӵĴ��ڣ�
��2����ɫ�����������Ƶij������������������Ժ���Al3+��
��3����Ba��OH��2��Һ��Ӧ���ɵİ�ɫ����������İ�ɫ���������ᱵ����ԭ�ᾧˮ��������SO42-��
��4�����ݵ���غ��ԭ���غ�������ش�
��5���ٸ������ʷ�Ӧ���Ⱥ�˳��ȷ�����ӷ�Ӧ����ʽ��
����������Ba��OH��2��Һ��Ӧ�������������������������ᱵ������
��NH4Al��SO4��2?12H2O������Ba��OH��2 ��Ӧ�������ᱵ������ƫ�����ơ�һˮ�ϰ���
��2����ɫ�����������Ƶij������������������Ժ���Al3+��
��3����Ba��OH��2��Һ��Ӧ���ɵİ�ɫ����������İ�ɫ���������ᱵ����ԭ�ᾧˮ��������SO42-��
��4�����ݵ���غ��ԭ���غ�������ش�
��5���ٸ������ʷ�Ӧ���Ⱥ�˳��ȷ�����ӷ�Ӧ����ʽ��
����������Ba��OH��2��Һ��Ӧ�������������������������ᱵ������
��NH4Al��SO4��2?12H2O������Ba��OH��2 ��Ӧ�������ᱵ������ƫ�����ơ�һˮ�ϰ���
���
�⣺��1��������һ����μ���NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����������ݳ����������д̼�����ζ����ʹʪ��ĺ�ɫʯ����ֽ������
�������ǰ�����֤��һ������笠����ӣ�
�ʴ�Ϊ��NH3��NH4+��
��2��������һ����μ���NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������࣬����ɫ�������ٲ�������ʧ��֤��һ�����������ӣ�
�ʴ�Ϊ��Al3+��
��3��һ�ݼ�������Ba��OH��2��Һ�����ɰ�ɫ���������ˣ���ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����46.6g�����ɫ����ΪBaSO4��˵��������������ӣ�
�ʴ�Ϊ��BaSO4��SO42-��
��4����������Ϣ��֪45.3g�ĸýᾧˮ�����У�n��SO42-��=
=0.2mol��n��NH4+��=
=0.1 mol���������ӻ����������������ӵ��ƽ���ԭ����n��NH4+��+3n��Al3+��=2n��SO42-���ɵã�n��Al3+��=
��2��0.2mol-0.1 mol��=0.1 mol��
n��H2O��=
=1.2mol��
����ԭ�ᾧˮ�������еĸ���������֮��Ϊ��m��NH4+����m��Al3+����m��SO42-��=��0.1mol��18g/mol������0.1 mol��27g/mol������0.2mol��96g/mol��
=6��9��64��
���ԣ�1.96g�ĸýᾧˮ�����У�n��NH4+����n��Al3+����n��SO42-����n��H2O��=0.1mol��0.1mol��0.2mol��1.2mol=1��1��2��12��
�ýᾧˮ����Ļ�ѧʽΪNH4Al��SO4��2?12H2O[��NH4��2SO4?Al2��SO4��3?24H2O]��
�ʴ�Ϊ��6��9��64��NH4Al��SO4��2?12H2O��
��5���ٸ��ݷ�Ӧ���Ⱥ�˳��ʼ�����������ƺ������ӷ�Ӧ�����������������Σ�Ȼ����笠����Ӻ����������ӷ�Ӧ���ɰ�����ˮ�ĽΣ�����������������������Ʒ�Ӧ����ƫ�����ƵĽΣ���NH4Al��SO4��2?12H2O���������������ʵ���֮��Ϊ1��2ʱ������ǡ�÷�Ӧ�������ᱵ������������һˮ�ϰ�����ʱ���ɳ�����������ӷ�Ӧ����ʽΪ��NH4++Al3++4OH-+2Ba2++2SO42-=2BaSO4��+Al��OH��3��+NH3��H2O��
�ʴ�Ϊ��NH4++Al3++4OH-+2Ba2++2SO42-=2BaSO4��+Al��OH��3��+NH3��H2O��
����������Ba��OH��2��Һ��Ӧ�������������������������ᱵ��������Ӧ�����ӷ���ʽΪ3Ba2++6OH-+3SO42-+2Al3+=3BaSO4��+2Al��OH��3����
�ʴ�Ϊ��3Ba2++6OH-+3SO42-+2Al3+=3BaSO4��+2Al��OH��3����
��NH4Al��SO4��2?12H2O������Ba��OH��2 ��Ӧ�������ᱵ������ƫ�����ơ�һˮ�ϰ�����Ӧ�����ӷ���ʽΪ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+NH3?H2O+AlO2-+2H2O��
�ʴ�Ϊ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+NH3?H2O+AlO2-+2H2O��
�������ǰ�����֤��һ������笠����ӣ�
�ʴ�Ϊ��NH3��NH4+��
��2��������һ����μ���NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������࣬����ɫ�������ٲ�������ʧ��֤��һ�����������ӣ�
�ʴ�Ϊ��Al3+��
��3��һ�ݼ�������Ba��OH��2��Һ�����ɰ�ɫ���������ˣ���ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����46.6g�����ɫ����ΪBaSO4��˵��������������ӣ�
�ʴ�Ϊ��BaSO4��SO42-��
��4����������Ϣ��֪45.3g�ĸýᾧˮ�����У�n��SO42-��=
| 46.6g |
| 233g/mol |
| 2.24L |
| 22.4L/mol |
| 1 |
| 3 |
n��H2O��=
| 45.3g-0.1mol��27g/mol-0.1mol��18g/mol-0.2mol��96g/mol |
| 18g/mol |
����ԭ�ᾧˮ�������еĸ���������֮��Ϊ��m��NH4+����m��Al3+����m��SO42-��=��0.1mol��18g/mol������0.1 mol��27g/mol������0.2mol��96g/mol��
=6��9��64��
���ԣ�1.96g�ĸýᾧˮ�����У�n��NH4+����n��Al3+����n��SO42-����n��H2O��=0.1mol��0.1mol��0.2mol��1.2mol=1��1��2��12��
�ýᾧˮ����Ļ�ѧʽΪNH4Al��SO4��2?12H2O[��NH4��2SO4?Al2��SO4��3?24H2O]��
�ʴ�Ϊ��6��9��64��NH4Al��SO4��2?12H2O��
��5���ٸ��ݷ�Ӧ���Ⱥ�˳��ʼ�����������ƺ������ӷ�Ӧ�����������������Σ�Ȼ����笠����Ӻ����������ӷ�Ӧ���ɰ�����ˮ�ĽΣ�����������������������Ʒ�Ӧ����ƫ�����ƵĽΣ���NH4Al��SO4��2?12H2O���������������ʵ���֮��Ϊ1��2ʱ������ǡ�÷�Ӧ�������ᱵ������������һˮ�ϰ�����ʱ���ɳ�����������ӷ�Ӧ����ʽΪ��NH4++Al3++4OH-+2Ba2++2SO42-=2BaSO4��+Al��OH��3��+NH3��H2O��
�ʴ�Ϊ��NH4++Al3++4OH-+2Ba2++2SO42-=2BaSO4��+Al��OH��3��+NH3��H2O��
����������Ba��OH��2��Һ��Ӧ�������������������������ᱵ��������Ӧ�����ӷ���ʽΪ3Ba2++6OH-+3SO42-+2Al3+=3BaSO4��+2Al��OH��3����
�ʴ�Ϊ��3Ba2++6OH-+3SO42-+2Al3+=3BaSO4��+2Al��OH��3����
��NH4Al��SO4��2?12H2O������Ba��OH��2 ��Ӧ�������ᱵ������ƫ�����ơ�һˮ�ϰ�����Ӧ�����ӷ���ʽΪ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+NH3?H2O+AlO2-+2H2O��
�ʴ�Ϊ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+NH3?H2O+AlO2-+2H2O��
���������⿼�����ӵļ�����ƶϡ����ӷ���ʽ����д����Ŀ�Ѷ��еȣ�ע����غ�ĽǶ��ƶϽᾧˮ����Ļ�ѧʽ��ע���������ӷ���ʽ����дԭ����ȷ�жϷ�Ӧ�����ӵĹ����������д���ӷ���ʽ�Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
����������ȷ���ǣ�������
A��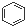 �� ��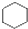 ���Ƿ������� ���Ƿ�������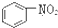 ���Ƿ��������Ƿ��㻯���� ���Ƿ��������Ƿ��㻯���� |
B�� �� �� ����������һ��-CH2-�������ͬϵ���ϵ ����������һ��-CH2-�������ͬϵ���ϵ |
| C����Ȼ����������������ú������Ҫ�ɷֶ��Ǽ��� |
| D������ʽΪC4H10O�����ʣ��������ڴ�������� |
���е���ʽ�У���ȷ���ǣ�������
A�� |
B�� |
C�� |
| D����N������N�� |
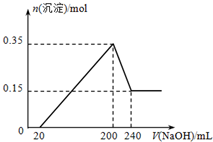 ��һ��������þ���������Ͷ��200mL�����У�����ȫ���ܽ����������Һ�м���5mol?L-1 NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ����ͨ������ش�
��һ��������þ���������Ͷ��200mL�����У�����ȫ���ܽ����������Һ�м���5mol?L-1 NaOH��Һ�����ɳ��������ʵ���n�����NaOH��Һ�����V�ı仯��ͼ��ʾ����ͨ������ش� ��֪��25�桢1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺
��֪��25�桢1.013��105Pa�£�1mol������ȫȼ������Һ̬ˮ�ų�285kJ����������ش��������⣺