��Ŀ����
8����ҵ��������������������Ҫ�ɷ�ΪFe2O3��FeO��SiO2�ȣ�Ϊԭ���Ʊ��ߵ��������죨Fe2O3�������������������£�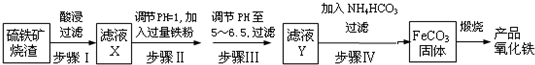
�Իش��������⣺
��1����ҺX�к��еĽ�����������Fe2+��Fe3+�������ӷ��ţ���
��2��������п�ѡ��B�Լ�������Һ��pH������ĸ����
A��ϡ���� B����ˮ C������������Һ D�����������Һ
��3�����˲����г��˲��������ձ�����Ҫ�IJ���������©����
��4��������ķ�Ӧ�¶�һ���������35�����£���Ŀ���Ƿ�ֹNH4HCO3�ֽ⣬����Fe2+��ˮ�⣮
��5���ڿ���������FeCO3���ɲ�Ʒ�������Ļ�ѧ����ʽΪ4FeCO3+O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+4CO2��
���� ��������������������������������������������˺�õ�������Ϊ�������裬��Һ�м���������ۣ���������������Һ������pH�����̼�������Һ��������̼���������壬��ϴ�ӡ�����ڿ���������ʱ�����ֽ⣬��������е���������������ԭ��Ӧ����������������
��1��������������Ҫ�ɷ�ΪFe2O3��FeO��SiO2�ȣ����������������������ᷴӦ�ܽ⣬�������費���ᷴӦ�����жϣ�
��2�����ݲ����������������Һ��������Ϊ�������ӣ����������Լ�������ҺPH��5-6.5���Լ����������������ӣ������Ǽ���ǿ����Һ��
��3�����ݹ��˲������õ����������⣻
��4���¶ȹ���̼����炙�ֽ⣬�¶����ߴٽ��������ӵ�ˮ�⣻
��5������������̼����������̼��������������Ӧ�����������Ͷ�����̼��ԭ���غ���ƽд����
��� �⣺��������������������������������������������˺�õ�������Ϊ�������裬��Һ�м���������ۣ���������������Һ������pH�����̼�������Һ��������̼���������壬��ϴ�ӡ�����ڿ���������ʱ�����ֽ⣬��������е���������������ԭ��Ӧ����������������
��1��������������Ҫ�ɷ�ΪFe2O3��FeO��SiO2�ȣ������������������ᷴӦ�ܽ⣬�������費���ᷴӦ���������ܽ�����������ӣ����������ܽ�������������ӣ�
�ʴ�Ϊ��Fe2+��Fe3+��
��2���ɲ����������������Һ��������Ϊ�������ӣ����������Լ�������ҺpH��5-6.5���Լ����������������ӣ�ǿ������Һ������������ӣ�
A��ϡ����������������������������ӣ���A�����ϣ�
B����ˮ��������Ե�����ҺPH����B���ϣ�
C������������Һ ��ǿ����Һ�����������������ӣ���C�����ϣ�
D�����������Һ�����������ԣ��������������ӣ���D�����ϣ�
��ѡB��
��3�����˲������õ��������в��������ձ���©����
�ʴ�Ϊ��©����
��4��������ķ�Ӧ�¶�һ���������35�����£��¶ȹ���̼����炙�ֽ⣬�¶����ߴٽ��������ӵ�ˮ�⣬
�ʴ�Ϊ����ֹNH4HCO3�ֽ⣬����Fe2+��ˮ�⣻
��5������������̼����������̼��������������Ӧ�����������Ͷ�����̼����ԭ���غ���ƽ��Ӧ�Ļ�ѧ����ʽΪ4FeCO3+O2 $\frac{\underline{\;����\;}}{\;}$ 2Fe2O3+4CO2��
�ʴ�Ϊ��4FeCO3+O2 $\frac{\underline{\;����\;}}{\;}$ 2Fe2O3+4CO2��
���� ���⿼���������仯�������ʵķ���Ӧ�ã���Ҫ�����̷�������ͷ�Ӧ���̵��жϣ���Ŀ�Ѷ��еȣ�

 �������ϵ�д�
�������ϵ�д�| A�� | ��ʳ�ɳ�ȥ��ˮ���ڱڵ�ˮ�� | |
| B�� | 75%��������������Ҵ���Һ������ҽ������ | |
| C�� | 35%-40%��ȩ��Һ�׳�Ϊ�������֣����������ݺ���Ʒ������Ч�� | |
| D�� | �ý��ݹ����������Һ�Ĺ�������ˮ���ͷŵ���ϩ���ɴﵽˮ�����ʵ�Ŀ�� |
| Ԫ�� | �����Ϣ |
| X | �����ڶ���ͬ�������壬������������ͷ���缫�����ײ��� |
| Y | �������������ڲ��������3�� |
| Z | ��������������������ˮ���������ǿ |
| W | ��XԪ�ش���ͬһ���壬��ҵ�ϳ���X�ĵ��ʻ�ԭW����������ȡW���� |
| A�� | ԭ���Ӱ뾶��W��Z��Y��X | |
| B�� | Y��Z����Ԫ��ֻ���γɻ�����Z2Y | |
| C�� | ��ͼ���̬�⻯������ȶ��ԣ�W��X | |
| D�� | ����������Ӧˮ��������ԣ�X��W |
| A�� | �ڷŵ�ʱ���õ�صĸ���������Ǧ�� | |
| B�� | �ڳ��ʱ��Ǧ���صĸ�����������Դ�ĸ������� | |
| C�� | �ڷŵ�ʱ�����·�е�������Ϊ�����������·������ | |
| D�� | �ڳ��ʱ�����������ķ�ӦΪ��PbSO4+2e-�TPb+SO${\;}_{4}^{2-}$ |
| A�� | �ڽ��������У��Ƶ��۵�ϵ� | |
| B�� | ���ڷ�����ѧ��Ӧʱ���ϼ����� | |
| C�� | ���ڿ�����ȼ�����������ƣ���������ɫ���� | |
| D�� | ��ԭ�������ֻ��һ�����ӣ�����ʧ���� |
| A�� | ���۹��塢Һ�廹�������ȼ�շ�Ӧ�����ǡ�H��O | |
| B�� | ���ȷ�Ӧ���淴Ӧһ���Ƿ��ȷ�Ӧ | |
| C�� | ̼����ȫȼ��ʱ������CO2�ų��������Ȳ���ȫȼ������CO�ų��������� | |
| D�� | H2��һ���������ɫ��Դ������Ϊȼ��1molH2���ų��������ر�� |
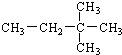 2��2-����-����
2��2-����-����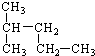 2-��-���飮
2-��-���飮 ijУ��ѧ�о���ѧϰС�飬��ѧϰ�˽�����֪ʶ��̽��Cu�ij������������ʣ��������£�
ijУ��ѧ�о���ѧϰС�飬��ѧϰ�˽�����֪ʶ��̽��Cu�ij������������ʣ��������£�