��Ŀ����
���ȸʯ��һ�ֺ�ͭ�Ŀ�ʯ����ͭ��̬Ϊ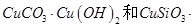
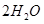 ��ͬʱ����
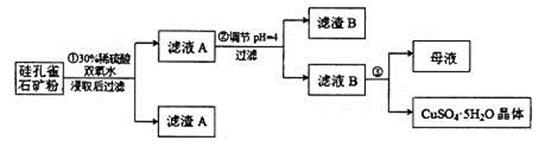
��ͬʱ����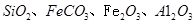 �����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
�����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
��ش��������⣺
��1����ɲ������ϡ������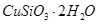 ������Ӧ�Ļ�ѧ����ʽ
������Ӧ�Ļ�ѧ����ʽ
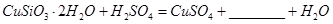 ��
��
�����ӷ���ʽ��ʾ˫��ˮ������_____________________________��
��2������ڵ�����ҺpHѡ�õ�����Լ���__________________
��3���й��������↑ʼ��������ȫ������pH���±���
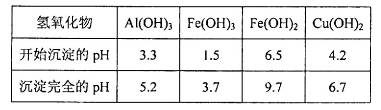
���ϱ���֪������ҺpH=4ʱ��������ȫ��ȥ��������______��������ȫ��ȥ��������________��
��4����ҺBͨ������Ũ���������Ũ��Ϊԭ����һ�룩����ȴ�ᾧ���Եõ�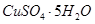 ���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�
���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�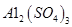 ������Һ��
������Һ��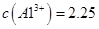 mol
mol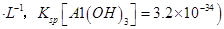 ______________��
______________��
��5����Ҫ�ⶨ����ͭ�����нᾧˮ�ĺ�������Ҫ�������Ǿƾ��ơ�������ƽ�����Ǽܡ������ǡ���������������������ǯ���в���ҩ�ס�_________________��ʵ�����������ͭ�������ʧˮ���ڿ�����ȴ���������ⶨ���______________(�ƫ�ߡ��� ��ƫ�͡����䡱)��
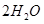 ��ͬʱ����
��ͬʱ����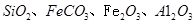 �����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
�����ʡ��Թ��ȸʯΪԭ����ȡ����ͭ�Ĺ�����������ͼ��
��ش��������⣺
��1����ɲ������ϡ������
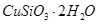 ������Ӧ�Ļ�ѧ����ʽ
������Ӧ�Ļ�ѧ����ʽ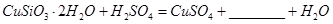 ��
�������ӷ���ʽ��ʾ˫��ˮ������_____________________________��
��2������ڵ�����ҺpHѡ�õ�����Լ���__________________
A��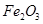 | B��CuO | C��A12O3 | D��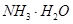 |

���ϱ���֪������ҺpH=4ʱ��������ȫ��ȥ��������______��������ȫ��ȥ��������________��
��4����ҺBͨ������Ũ���������Ũ��Ϊԭ����һ�룩����ȴ�ᾧ���Եõ�
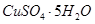 ���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�
���塣ijͬѧ��Ϊ������������������������������������������ݶԸ�ͬѧ�Ĺ۵��������ۣ���֪�����£�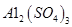 ������Һ��
������Һ��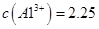 mol
mol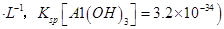 ______________��
______________����5����Ҫ�ⶨ����ͭ�����нᾧˮ�ĺ�������Ҫ�������Ǿƾ��ơ�������ƽ�����Ǽܡ������ǡ���������������������ǯ���в���ҩ�ס�_________________��ʵ�����������ͭ�������ʧˮ���ڿ�����ȴ���������ⶨ���______________(�ƫ�ߡ��� ��ƫ�͡����䡱)��
��1��H4SiO4��1�֣� 2Fe2++H2O2+2H+= 2Fe3++2H2O��2�֣�
��2��B��2�֣�
��3��Fe3+��2�֣���Al3+��2�֣�
��4����ͬѧ�Ĺ۵��Ǵ���ģ�1�֣�ͨ�������֪����ҺB��C��Al3+��=3.2��10-4 mol?L-1,Ũ����C��Al3+��=6.4��10-4 mol?L-1��2.25 mol?L-1�����Բ���������������ϴ������2�֣�
��5��������1�֣�ƫ�ͣ�1�֣�
�����������1������ԭ���غ��֪ȱH4SiO4����ȸʯ���ܺ���Fe2+��˫��ˮ��ǿ�����ԣ�����������ԭ��Ӧ�� 2Fe2++H2O2+2H+= 2Fe3++2H2O����2������pH=4��Ŀ���dz���Fe3+�����õ�����ͭ���壬Ϊ���������ʣ�ѡ��B����3����ϱ����������ݷ�������4����ͬѧ�Ĺ۵��Ǵ���ģ�ͨ�������֪����ҺB��C��Al3+��=3.2��10-4 mol?L-1,Ũ����C��Al3+��=6.4��10-4 mol?L-1��2.25 mol?L-1�����Բ���������������ϴ������5������������ͭ�ڿ���������ȴ�������˲���ˮ��������������ýᾧˮ����ƫ�͡�

��ϰ��ϵ�д�
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ
 2Cu��O2����4H��
2Cu��O2����4H��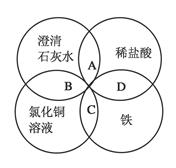
 2I-+SO42��+4H+
2I-+SO42��+4H+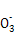 =4Mg2++N
=4Mg2++N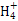 +3H2O
+3H2O
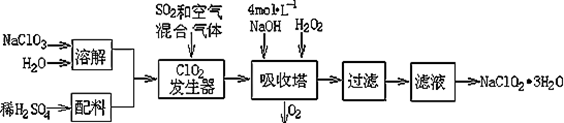
 +2H2O
+2H2O 4OH-+O2��
4OH-+O2�� +4H+
+4H+
 +2H+ =CO2��+H2O
+2H+ =CO2��+H2O =" " BaSO4��
=" " BaSO4��